Showing Results for Energy
Search term may appear only in full report available to members. Join now for full access.
CL Answer
Which supplements help to improve energy and decrease fatigue?
Energy supplements and vitamins - Some can help increase energy and reduce fatigue, including CoQ10, curcumin/turmeric, cocoa, and B vitamins.

Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Which B Vitamins Are ConsumerLab's Top Picks!

Product Review
Nutrition Bars & Cookies Review (For Energy, Fiber, Protein, Meal Replacement, and Whole Foods)
Find the Best Nutrition Bar or Cookie. ConsumerLab Tests Reveals Not All Nutrition Bars and Cookies Contain What They Claim.

CL Answer
Can taking too much vitamin B-12 be dangerous? The label on my B-complex states it contains 50,000% of the Daily Value!
Find out the daily value for vitamin B-12 and the potential side effects of taking too much B-12. ConsumerLab explains why taking too much vitamin B-12 can be harmful.
CL Answer
Is there a risk of liver toxicity with certain supplements?
Find out if there is risk of liver damage from supplements such as green tea, niacin, red yeast rice and vitamin A.
Product Review
Ginseng Supplements Review
Find the Best Ginseng Supplement. Key "Ginsenosides" Found to Range 10-Fold Across Products.

CL Answer
Can taking too much vitamin B-6 be dangerous?
Find out if multivitamins contain excessive amounts of B-6, how much B-6 is too much, and symptoms of toxicity and overdose.

Product Review
NAD Booster Supplements Review (NAD+/NADH, Nicotinamide Riboside, and NMN)
How Important Is Boosting NAD+ Levels? Find Out and Learn How Booster Supplements Compare.
CL Answer
Does nicotinamide riboside (as in Niagen and Basis) really have anti-aging or other health benefits?
Niagen (Nicotinamide riboside) information, including if it actually provides anti-aging or other health benefits. ConsumerLab explores this question.
News Release
April 11, 2024
Best CoQ10 & Ubiquinol Supplements According to ConsumerLab Tests
White Plains, NY, April 11, 2024 — CoQ10 and its active form, ubiquinol, are among the most popular supplements, taken by 42.2% of supplement users, often taken by people on cholesterol-lowering statin medication and for a variety of other conditions.
Product Review
Coconut Oil and Medium Chain Triglycerides (MCT) Oil Review — Semi-Solid and Liquid Oils & Supplements
Find the Best Coconut Oil and MCT Oil. See How These Oils Compare on Medium Chain Triglycerides (MCTs), Quality, and Value.

CL Answer
Which air purifiers are best for reducing the spread of COVID-19?
Find out if portable air cleaners can remove the virus that causes COVID-19 infection from the air and learn which type air cleaners work best.

CL Answer
Should I be concerned about phytic acid in oat cereals? I've heard that it can be harmful, but also that it can have health benefits.
Get information on phytic acid levels found in oats and oat cereals.

Product Review
Electrolytes & Sports Drinks Review
See Which Sports Drinks, Powders, and Pills Deliver the Right Electrolytes
CL Answer
I thought the B vitamins were all water soluble and did not build up in the body, so you would not build up toxic levels. Am I wrong?
Learn more about water-soluble B vitamins including niacin, B6, B12 and folic acid, and why taking too much of these vitamins can be toxic. See the symptoms of overdose and toxicity from B vitamins. ConsumerLab.com's answer explains.

Clinical Update
2/16/2019
Energy Drinks & Aggression
Are people who frequently consume energy drinks (like 5-hour Energy) more likely to be aggressive or have mental health problems? Learn what a new study found, and learn more about the pros and cons of energy drinks in the Energy Drinks section of the B Vitamin Supplements Review.
Product Review
Rhodiola Rosea Supplements Review
Do Rhodiola Supplements Help With Depression and Anxiety? Find Out and See Which Rhodiola Supplements Provide the Best Quality & Value.

CL Answer
I'm over 50 and looking to take a vitamin B-12 supplement. Many contain a form of vitamin B-12 called cyanocobalamin, yet I read on the Internet that this form is toxic. Should I be concerned?
Looking to see if the cyanocobalamin form of vitamin B-12 is safe? Read research and safety information on ConsumerLab's report.

Product Review
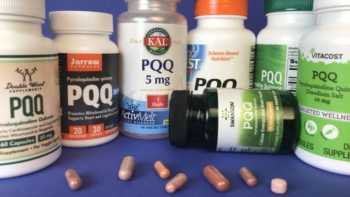
PQQ (Pyrroloquinoline Quinone) Supplements Review
Learn What PQQ Does and Which PQQ Supplements are Best
CL Answer
Does Gynostemma pentaphyllum really work as an AMPK activator and help for diabetes, high cholesterol, or weight loss?
Find out if Gynostemma pentaphyllum ( jiaogulan) works as an AMPK activator and helps to lower blood sugar and treat diabetes. ConsumerLab.com's answer explains.

CL Answer
Adrenal Fatigue and Adrenal Support Supplements: Do they work and are they safe?
Find out what's really in supplements for adrenal support and adrenal fatigue, including herbal adrenal support supplements and bovine derived adrenal support supplements. Find out if they really work and if they are safe. ConsumerLab.com's answer explains.

CL Answer
What do alkaline booster drops do? Do they provide the same benefits as Kre-Alkalyn?
Learn more about alkaline drop supplements and what booster drops and KreAlkalyn are marketed to do.

Product Review
Kelp Supplements Review
Choose the Best Kelp Supplement. Be Cautious With Kelp! Only 50% of Supplements Pass CL's Review.

CL Answer
Is it okay to take vitamin D3 and fish oil together?
Find out if taking vitamin D and fish oil is okay, how it effects absorption, and the best way to take vitamin D to improve absorption. ConsumerLab.com's answer explains.

Clinical Update
11/18/2012
Caution with Energy Drinks
The FDA has released reports showing deaths and other serious adverse events associated with the use of energy drinks like 5-hour Energy. As ConsumerLab.com reported in September, these small "shot" drinks can pack more caffeine than 2 cups of regular coffee. While the caffeine can keep you alert, there are a number of concerns to bear in mind and suggestions on how to more safely use these drinks. Learn more in the update to our B Vitamins and Energy Drinks Review. More >>
Clinical Update
11/17/2014
Keep Energy Drinks Away from Kids
It was reported this week that 40% of calls to poison centers for "energy drink exposure" involved children under age 6, many of whom experienced serious side effects. For more about this and other concerns with energy drinks, as well as our test results for popular products, see the B Vitamins & Energy Drinks Review >>
Product Review
CoQ10 and Ubiquinol Supplements Review
Find the Best CoQ10 and Ubiquinol Supplements and Learn How They Differ

CL Answer
What is Marine-D3 with Seanol-P, and does it live up to anti-aging claims?
Learn about the evidence for Marine-D3, a supplement from Marine Essentials that contains Seanol, including evidence from clinical studies on aging.

CL Answer
Bee pollen: Purported health benefits and possible side effects
Bee pollen is promoted for numerous health conditions, including supporting immune health and boosting energy. Find out if it works and if it's safe.

Recalls & Warnings
January 03, 2024
FDA Warns Amazon for Selling Men’s Supplements Containing Prescription Drugs
On December 20, 2023, the FDA issued a Warning Letter to Amazon.com, Inc.
CL Answer
Can eating almonds reduce wrinkles?
Find out if eating almonds can reduce wrinkles or improve aging skin.

CL Answer
Butyrate Supplements for Gut Health: Does butyric acid really work and is it safe?
Can butyrate supplements improve gut health or help reduce symptoms of irritable bowel syndrome (IBS) or Crohn's disease? Find out what the clinical evidence shows, learn about the different forms of butyrate, including sodium butyrate, calcium-magnesium butyrate, and tributyrate, and get details about products such as BodyBio Sodium Butyrate and Healus Complete Biotic Tributyrin, dosage, safety and cost.

CL Answer
Are lozenges and sublingual pills considered dietary supplements?
Learn more about lozenges and sublingual pills (such as echinacea, zinc and B12) including safety warnings.

CL Answer
Is MitoQ a better form of CoQ10?
MitoQ information, including how it is absorbed in the body and how it compares to CoQ10.

CL Answer
Is P-5-P really better than "regular" vitamin B6?
Learn more about pyridoxal-5-phosphate (P-5-P) and vitamin B6.

News Release
November 02, 2023
Spirulina and “Greens” Supplement Tests Show Some Problems
White Plains, New York, November 2, 2023 — ConsumerLab tests of spirulina, chlorella, and “greens” supplements revealed that tablets of three brands of spirulina failed to disintegrate within the allotted time, and several other products contained amounts of lead unsuitable for regular consumption ...
Product Review
Seaweed Snacks and Foods Review
Tests Reveal Seaweed Snack Risks

Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

CL Answer
What are the health benefits of D-ribose and is it safe?
Find out if D-ribose supplementation is beneficial for heart failure, exercise performance, and fibromyalgia and learn if it is safe.

CL Answer
Is sublingual vitamin B-12, which is placed under the tongue and allowed to dissolve, really better than the pill form?
Learn the difference between sublingual vitamin B-12, vitamin B-12 pills and more through information including clinical evidence.

CL Answer
Which oils can help lower my cholesterol and risk of heart attack? How are coconut oil, olive oil, and fish oil for example?
Find out which fats and oils, such as coconut oil and fish oil, lower cholesterol and lower the risk of cardiovascular disease.

Clinical Update
2/10/2021
Energy Drinks and Blood Pressure
Which people are more likely to have cardiovascular side effects from energy drinks? Find out what a new study showed in the Energy Drinks & Shots section of our B Vitamins Supplements Review.
Clinical Update
10/08/2014
Energy Drinks: Athletic Performance & Side Effects
A recent study found that a caffeinated energy drink improved athletes' self-perceived muscle power, but also increased insomnia and nervousness. Get the details in the update to the B Vitamin Supplements and Energy Drinks Review >>
CL Answer
Are the "% DV" numbers on vitamin supplement labels really based on what I need?
Learn more about percent daily values, including if they are good standards of measure or if better upper and lower limits exist.

CL Answer
The maker of my multivitamin says it doesn't include folic acid because too much from supplements can be harmful. Is that true?
Learn more about the harmful effects from too much folic acid, including increased risk of prostate cancer and kidney damage.

CL Answer
Do "Mr. Happy Stack" supplements improve memory, cognition and mood, and are they safe?
Find out if Happy Stack supplements really work to enhance memory and cognition, plus safety and side effects. ConsumerLab.com's answer explains.
CL Answer
Does urolithin A reduce age-related muscle decline or improve osteoarthritis?
Find out if urolithin A may improve age-related muscle decline and learn about its safety.

Product Review
Muscle & Workout Supplements Review (Creatine and Branched-chain Amino Acids)
Do Creatine and BCAAs Really Improve Strength and Recovery?

Clinical Update
10/09/2021
Niagen for Energy?
The maker of Niagen (a supplement containing nicotinamide riboside) was challenged regarding its claim that Niagen boosts energy and performance. See how the company responded in the Nicotinamide Riboside section of our B Vitamins Supplements Review.
Clinical Update
12/14/2017
Danger from Too Much Vitamin B6
A women who was consuming large amounts of an energy drink as well as a B-vitamin complex developed symptoms of vitamin B6 (pyridoxine) toxicity (which may not be reversible), according to a recent report. Get the details in the "B6" section of B Vitamin Supplements Review >>
CL has long warned that energy drinks and shots often contain large amounts of B6 and other B vitamins.
Clinical Update
5/26/2023
D-Ribose for Energy?
D-ribose is promoted for increasing energy during exercise and reducing fatigue in people with fibromyalgia. But does it really work, and is it safe? Find out in our article about the health benefits of D-ribose.
CL Answer
How can I avoid aconite – a toxin – in Aconitum supplements?
Aconite is a highly toxic alkaloid found in Aconitum plants ( also called monkshood, wolfsbane, or devil's hood). Aconite poisoning can cause low blood pressure, chest pain, increased or decreased heart rate, and irregular heart rhythm that can lead to cardiac arrest and death. Find out which supplements may contain aconite and how to avoid it.

CL Answer
Which supplements can help treat anemia? Do any supplements cause a low red blood cell count?
Find out which vitamin and nutrient supplements can help prevent and treat anemia in people who are nutrient deficient and learn which supplements may cause low blood cell counts, especially when used in excess.

CL Answer
Can prenatal vitamins have too much folic acid? Mine has 800 mcg, but isn't that more than what's recommended? Is this dangerous to me or my baby?
Is it possible for prenatal vitamins to have too much folic acid? Find out the correct dosage so that it is not dangerous to babies.

CL Answer
Do vitamin patches, such as for B12 or multivitamins, really work? How about those from PatchMD?
Do vitamin B12 Patches like PatchMD really work? Can you absorb vitamin B12 through patches?
CL Answer
Which supplements reduce the risk of stroke? Which increase the risk of stroke?
Find out which supplements (may help reduce the risk of stroke/might increase the risk of stroke.)

CL Answer
What are the health benefits of coconut oil and medium chain triglycerides, such as those used in Bulletproof Coffee?
Learn the health benefits of coconut oil and medium chain triglycerides (MCTs), including evidence from Bulletproof Coffee, clinical studies on obesity, skin, and Alzheimer's disease.

CL Answer
What is C15:0 fatty acid (found in fatty15), and does it have health benefits?
C15:0 fatty acid in fatty15 is promoted for “healthier hair & skin, balanced metabolism, and deeper sleep,” as well as “slowing the aging process.” Find out if it works.

CL Answer
Are intravenous (IV) vitamin infusions safe and effective?
Intravenous (IV) vitamin infusions are touted by celebrities and promoted by wellness clinics for numerous conditions. Find out if they work and if they are safe.

Clinical Update
7/19/2014
High-Dose Niacin with Statins Not Worth It
Further evidence was published this week showing that the addition of high-dose niacin (vitamin B-3) to cholesterol-lowering treatment with statin drugs provides no worthwhile benefit and appears to increase the risk of adverse side events. For details, see the Review of B Vitamin Supplements and Energy Drinks >>
Clinical Update
4/14/2013
Concern Over Carnitine Causing Cardiovascular Disease
Intake of L-carnitine from supplements (and from red meat) may foster atherosclerosis (hardening of the arteries), according to new research. Once ingested, L-carnitine is converted in some people to a compound which reduces the normal clearing of cholesterol from arteries.
For more details, see the updated reviews of B Vitamin Supplements & Energy Drinks and Protein Powders, as some of these products contain L-carnitine. The Acetyl-L-Carnitine Supplements review has also been updated because acetyl-L-carnitine is chemically similar to L-carnitine, suggesting a potential concern.
Clinical Update
8/06/2014
When Vitamin C May Curb a Cold
A recent small study among men with low to adequate levels of vitamin C showed that supplementing with vitamin C reduced the incidence of colds compared to placebo. The men taking vitamin C also reported having more energy -- although this finding was not statistically significant. Learn more about what vitamin C can and cannot do, the dose used in this study, and results of our tests of vitamin C supplements, in the update to the Vitamin C Supplements Review >>
Clinical Update
6/16/2013
Stimulant Supplements
Have you ever wondered what taking a stimulant supplement for weight management or energy might do to your body? Researchers recently gave Zantrex-3, Xenedrine EFX, Metabolift, or a brand of Guarana to young men while monitoring their heart rates and other vital signs, and recording side effects. The results raise concerns. Get the details, plus test results for 14 other supplements, in the updated Weight Supplements Review >>
Clinical Update
5/15/2018
Too Much Vitamin B6
Be aware that you can get too much vitamin B6 from supplements and energy drinks. This can result in nerve damage and other adverse effects, as has been recently reported. For details, see the B6 section of the B Vitamin Supplements Review.
Clinical Update
1/11/2022
Ginkgo and Cistanche for Chronic Fatigue Syndrome?
Does supplementing with ginkgo and cistanche extracts help reduce symptoms of chronic fatigue syndrome? See what a recent study showed in our updated answer to the question: Which supplements help to improve energy and decrease fatigue?
Clinical Update
3/23/2021
MCT Oil for Mild Cognitive Impairment?
Can medium chain triglycerides (MCTs – from coconut oil) boost brain energy and improve thinking in people with mild cognitive impairment? Find out what a recent study found in the What It Does section of our Coconut and MCT Oils Review. Also see our Top Picks among coconut and MCT oils.
Clinical Update
10/16/2020
Vitamin C for Vitality?
Can taking a vitamin C supplement or eating a vitamin C-rich food improve mood, energy, or overall well-being? Find out what a recent study showed in the What It Does section of the Vitamin C Supplements Review. Also see our Top Picks for vitamin C.
Clinical Update
1/12/2024
L-Theanine for Fatigue?
Did drinking a beverage containing L-theanine, with or without CBD, reduce fatigue? See what a recent study showed in the Stress, sleep and cognition section of our L-Theanine Review, which includes our Top Picks for L-theanine.
Also see: Which supplements help to improve energy and decrease fatigue?
CL Answer
Is it true that eating Brazil nuts can help with weight loss? Are there other health benefits or risks of Brazil nuts
Learn more about vitamin E intake from nuts, including Brazil nuts, hazelnuts, and almonds.

CL Answer
What is CELLFOOD and is it a healthy supplement?
Learn more about CELLFOOD (NuScience Corporation), including if the claims to improve athletic performance, fibromyalgia, weight loss, and cancer are true.
CL Answer
What is Skinny Fiber and does it really work? I see that glucomannan is a key ingredient. What is that?
Learn more about Skinny Fiber, including its key ingredient glucomannan, evidence from clinical studies, dosage info, and safety.

CL Answer
Rhodiola and ashwagandha are both promoted to help with stress and anxiety. Is it safe to take them at the same time?
Find out if it is safe to take ashwagandha and rhodiola together. Both herbs are promoted to help with stress and anxiety, but may also lower blood pressure and have other side effects. ConsumerLab.com's answer explains.

CL Answer
Is kratom too dangerous to use?
Find out if kratom is safe/legal to use, including potential adverse effects, such as agitation, rapid heartbeat, nausea, drowsiness, seizures, and possible drug interactions.

CL Answer
Are Balance of Nature products good dietary supplements?
Do Balance of Nature supplements "Fruits" and "Veggies" supplements really provide the daily recommended servings of fruits and vegetables, and are they worth the cost? Find out.

Recalls & Warnings
August 25, 2023
FDA Warns Consumers Not to Use Big Guys Male Energy Supplement
On August 22, 2023, the FDA warned consumers not to buy or use BIG GUYS Male Energy Supplement after FDA laboratory analysis found it to contain undeclared sildenafil.
News Release
June 14, 2022
Best B Complexes, B12, and Other B Vitamin Supplements Revealed by ConsumerLab Tests: Beware of High Doses
White Plains, New York, June 14, 2022 — Recent ConsumerLab tests of over 30 popular vitamin B complexes and supplements revealed that four products contained either much less, or much more, of at least one B vitamin listed on the label.
CL Answer
What is desiccated beef liver? What is it used for, and is it safe?
Description of desiccated beef liver, including what it is used for, its nutrient content, and safety concerns.

CL Answer
Do any supplements naturally suppress appetite and help with weight loss?
Learn which natural ingredients can suppress appetite and find if these natural appetite suppressants help with weight loss.

CL Answer
What are trace minerals and do I need them?
Trace minerals include boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, zinc, and others. Find out what they do, how much of each trace mineral you should be getting, and who's most likely to be deficient in these minerals.
CL Answer
I have type 2 diabetes. Should I be concerned about ingredients like maltodextrin and maltitol that are found in some protein bars and drinks?
Learn more about maltitol and maltodextrin and their effects on blood glucose levels for people with type 2 diabetes.

CL Answer
Do sea moss and carrageenan have health benefits? Are there risks?
Sea moss is promoted for thyroid health, boosting immunity, improving gut health, and other uses. Find out if it works and learn about possible side effects of sea moss and its constituent, carrageenan.

Product Review
Multivitamin and Multimineral Supplements Review
Best Multivitamins In 2023 -- Caution with Gummies

CL Answer
Is there a danger in taking lecithin or phosphatidylcholine? I heard that they may increase the risk of heart attacks.
Lecithin, phosphatidylcholine and choline - Learn more about the potential link between them and cardiovascular disease.

CL Answer
Does I3C (from broccoli) or DIM (a related chemical) reduce the risk of cancer?
Find out if the compound indole-3-carbinol (I3C), from broccoli, and its metabolite diindolylmethane (DIM) can reduce the risk of cancer. ConsumerLab.com's answer explains.

CL Answer
Can beetroot juice improve my workout performance?
Find out if beetroot juice can help improve exercise endurance or performance, how it works, the clinical evidence, and cautions.. ConsumerLab.com's answer explains.

CL Answer
Does Alpha Brain really improve memory, focus and cognition?
Find out if Alpha Brain really improves memory, focus and cognition. See the clinical evidence and get details about Alpha Brain ingredients, as well as potential side effects and drug interactions. ConsumerLab.com's answer explains.

CL Answer
Is there a multivitamin that contains only the recommended daily vitamins and minerals? Most that I have looked at contain more.
Are there any multivitamins that contain only the recommended daily vitamins and minerals? Choose the best multivitamin based on age & gender.

News Release
February 09, 2022
Caution With Spirulina Supplements: ConsumerLab Tests Reveal Lead Contamination, Quality Concerns
White Plains, New York, February 9, 2022 — Problems were discovered in three out of four spirulina supplements recently tested by ConsumerLab. One product was found to be contaminated with lead, and two other products, which were tablets, failed to disintegrate within the expected time.
Recalls & Warnings
February 13, 2018
American College of Sports Medicine Warns of Energy Drink Dangers
A new statement released by the American College of Sports Medicine (ACSM) highlights the dangers of energy drinks when consumed by children and adolescents, and calls for more studies on the safety and efficacy energy drinks.
CL Answer
Do any supplements or lifestyle changes reduce the symptoms of tinnitus? Is it true that some supplements can cause tinnitus?
Learn which supplements can ease tinnitus, including melatonin and pine bark extract. Understand which may actually cause tinnitus.

CL Answer
Which supplements or foods can help lower cholesterol and keep my heart healthy? Are there any to avoid?
Information about heart health supplements & vitamins that help with cholesterol, including sterol esters, CoQ10, and vitamin D, and which may be bad for the heart, like calcium.

CL Answer
Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
Find out which supplements help improve memory, brain function and cognition, including fish oil, some B vitamins, cocoa, and curcumin. ConsumerLab's answer explains the evidence for supplements promoted to help with brain function and cognition.
Recalls & Warnings
January 16, 2018
Adverse Effects From Energy Drinks Common Among Youth and Young Adults
More than half (55.4%) of young people who have ever consumed an energy drink have experienced at least one adverse reaction, according to study published yesterday in CMAJ Open, a journal of the Canadian Medical Association.
News Release
November 05, 2021
NAD Boosters for Energy and Memory? ConsumerLab Reviews NAD+, NAHD and NMN Supplements
White Plains, New York, November 5, 2021 — Most cells in the body use NAD (nicotinamide adenine dinucleotide) in the process of energy production, and compounds that boost blood levels of NAD are often sold in supplements marketed for a wide range of benefits, from increasing energy and ...
Product Review
Alpha Lipoic Acid Supplements Review
Choose the Best Alpha-Lipoic Acid Supplement — See the Amounts of Active "R-Isomer" We Found

News Release
September 11, 2021
Best CoQ10 and Ubiquinol Supplements Identified in Testing by ConsumerLab
White Plains, New York, September 11, 2021 — CoQ10 and ubiquinol, the active form of CoQ10, are often taken to offset a decline in natural levels that occurs with the use of statin (cholesterol-lowering) medication, and are also promoted for increasing energy and anti-aging effects.
News Release
June 16, 2021
Wide Variations in Ginseng Potency, Quality Revealed by ConsumerLab Tests
White Plains, New York, June 16, 2021 — Ginseng is promoted for a wide range of uses, from increasing energy, "vitality," and libido, to improving blood sugar levels and preventing colds.
Recalls & Warnings
June 20, 2019
Weight Loss, Muscle & Energy Supplements Linked to Adverse Events in Children and Young Adults
Consumption of dietary supplements sold for weight loss, muscle building, and energy are associated with an increased risk for severe medical events in children and young adults compared to the consumption of vitamins, according to a recent study published in the Journal of the Adolescent ...
CL Answer
Top 8 vitamins and nutrients for vegetarians and vegans
Find out which 8 supplements may be needed by vegetarians or vegans to meet all their daily nutrient requirements and learn ways to find supplements that do not contain ingredients objectionable to people following vegetarian or vegan diets.

Recalls & Warnings
August 16, 2013
Maker of Energy, Weight Loss, Sleep and Vitamin D Supplements Warned for Manufacturing Violations
On July 22, 2013, the FDA issued a warning letter to N.V.E. Pharmaceuticals, Inc.
Recalls & Warnings
May 22, 2023
Nature’s Energy Children’s Colostrum Recalled Due to Undeclared Milk
On May 18, 2023, Nature’s Energy issued a recall of six lots of Children’s Chewable Colostrum products due to undeclared milk.
CL Answer
Which vitamins and supplements should be stopped before getting blood work and laboratory tests?
Find out which vitamins and supplements can interfere with blood, urine or stool tests, and imaginging tests. Learn how B vitamins such as biotin, niacin and riboflavin, as well as calcium supplements, St. John’s wort, vitamin C, and others supplements can affect test results, as well as common foods that may interfere with tests.
Recalls & Warnings
June 23, 2020
BioPure Healing Products Warned for Manufacturing Violations
On June 17, 2020, the FDA issued a warning letter to BHP Holdings Inc.
Recalls & Warnings
November 17, 2022
FDA Warns Consumers Not to Use Male Enhancement Supplement
On November 10, 2022, the FDA warned consumers not to buy or use Sangter Natural Male Energy Supplement after FDA laboratory analysis confirmed the presence of undeclared sildenafil.
CL Answer
Which supplements and lifestyle changes improve sleep, and which cause insomnia?
Find out which vitamins may help you sleep and learn if any vitamins or supplements can cause insomnia.

CL Answer
What are human growth hormone (HGH) supplements? What are the benefits? Are they safe?
Learn about the benefits and risks of human growth hormone (HGH) supplements and injections. Get science-based insights on usage and effects.
CL Answer
What is the difference between CoQ10 and ubiquinol?
Understand the difference between CoQ10 (ubiquinone) and the active form, ubiquinol, and how they are best absorbed. ConsumerLab explains.

CL Answer
Which supplements help with erectile dysfunction?
Supplements for erectile dysfunction. Find out which supplements can help with ED, including ginseng, L-arginine, and maca.

CL Answer
What supplements should I stop taking before surgery?
Info on which supplements to avoid before surgery due to increased risk of bleeding or interference with anesthesia.
CL Answer
Do reishi mushroom supplements boost the immune system, or have other health benefits?
Learn more about the health benefits of reishi mushroom extract supplements, including evidence from clinical studies on cancer and diabetes, plus information about safety and price.

CL Answer
What are the health effects of turkey tail mushroom and is it safe?
Learn about the possible health effects of turkey tail mushroom, including its use as adjuvant treatment for cancer and for boosting immune function, plus get information about possible side effects and find out why ConsumerLab has not yet tested turkey tail products.

Recalls & Warnings
November 04, 2016
Energy Drink Linked to Case of Acute Hepatitis
A case of acute hepatitis has been linked to daily consumption of energy drinks, according to a report published on November 1, 2016, in BMJ Case Reports.
Recalls & Warnings
January 24, 2020
Fat-Burning, Energy Supplement Linked to Heart Trouble
A 33-year-old woman in Australia developed cardiac ischemia (a lack of blood flow to the heart) after taking the "fat burning" supplement Alpha Lean-7, according to a recent report in the Journal of Sports Sciences.
Recalls & Warnings
August 03, 2022
Sexual Enhancement Supplement Recalled Due to Sildenafil
On August 1, 2022, DISTRIBUTOR RFR, LLC recalled one lot of SANGTER Energy Supplement 3000 mg to the consumer level after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
CL Answer
I take levothyroxine (Synthroid), a thyroid hormone to treat hypothyroidism. Are there supplements or foods I should avoid, or be taking, due to this drug?
Is taking certain supplements and hypothyroidism medications bad? Learn which supplements can interact with thyroid hormone medications like levothyroxine (Synthroid), thyroid hormone levels, and laboratory tests.
Recalls & Warnings
May 04, 2023
FDA Warns MedoLife for Promoting Homeopathic Products to Treat COVID-19, Cancer
On April 19, 2023, the FDA issued a warning letter to Medolife Rx D/B/A MedoLife Corp., AELIA Inc. and Queanta Inc.
Recalls & Warnings
September 06, 2023
WEFUN Capsules Recalled Due to Undeclared Sildenafil
On August 25, 2023, WEFUN Inc. issued a recall of 300 boxes of WEFUN Capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
May 01, 2023
TruVision Recalls Products Due to Presence of Potentially Dangerous Ingredients
On April 27, 2023, TruVision Health issued a recall of various dietary supplement products because they contain hordenine and/or octodrine/DMHA, compounds that the FDA considers to be “possibly unsafe” and which are not permitted to be sold as dietary supplements.
Recalls & Warnings
January 11, 2023
Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 9, 2022, the FDA issued a warning letter to Distributor RFR, LLC after laboratory analysis of the company’s SANGTER Natural Male Energy Supplement found the product to contain undeclared sildenafil, a prescription medication.
News Release
September 11, 2019
Some Nutrition Bars Contain More Carbs and Less Fiber Than Listed, ConsumerLab Tests Reveal
White Plains, New York, September 11, 2019 — Nutrition bars and cookies are promoted as a convenient way to get protein, fiber and other nutrients on-the-go, but recent ConsumerLab tests of popular nutrition bars and cookies reveal that some contain more carbohydrates (as well as fat and ...
Recalls & Warnings
June 16, 2020
Seller of Silver, Arginine, and More Warned for Manufacturing Violations
On June 1, 2020, the FDA issued a warning letter to Morningstar Minerals LLC, which found the company's products, including Silver Boost, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
CL Answer
Do hair loss supplements, such as Viviscal, Hair La Vie, and Nutrafol, or topical essential oils work?
Vitamins and supplements that may help with hair loss and thinning, including saw palmetto, beta-sitosterol, protein, iron, and vitamin D.

Product Review
Astaxanthin Supplements Review
See Which Astaxanthin Products Passed or Failed CL's Tests

Recalls & Warnings
April 18, 2022
Moringa Concern: Blood Clots
A 63-year-old woman in New York with type 2 diabetes developed a blood clot in the lungs (i.e. pulmonary embolism) that her physicians suspect may have been caused by the use of a supplement containing Moringa oleifera extract (Ebhohon, Int J Emerg Med 2022).
CL Answer
When taking a statin drug like Lipitor or Crestor, are there supplements I should avoid or take?
Learn about the interactions between certain supplements and atorvastatin (Lipitor), rosuvastatin (Crestor), and other cholesterol-lowering statins.
CL Answer
Do any supplements help prevent or treat a cold?
Are there supplements that can help prevent or treat cold symptoms? Learn more about zinc, probiotics, vitamin C and more.

CL Answer
Is it true that there are bits of plastic in drinking water, specialty salts, or other foods and beverages? How concerned should I be?
Microplastics are tiny pieces of plastic that can end up foods and beverages. Learn about microplastics found in drinking water and specialty salts. ConsumerLab.com's answer explains.

CL Answer
What are CoQ10 side effects?
CoQ10 side effects, such as nausea, headache, and insomnia, and how you can reduce the occurrence of CoQ10 side effects. ConsumerLab.com's answer explains.

CL Answer
What is vitamin B12 and how much do I need?
Vitamin B12 dosage varies depending on your age, gender, and various other factors. Our CL Answer explains why B12 is important, the recommended dosages, deficiency, health benefits, and the best form.
CL Answer
Which supplements are important after bariatric surgery (i.e., weight loss or stomach-reducing surgery)? Are there any I should avoid?
Find out which supplements may help after weight loss or stomach reducing surgery, including iron and calcium, and which should be avoided.
CL Answer
What is Perque Bone Guard Forté? It is safe and effective for bone health?
Find out about the ingredients in Perque Bone Guard Forté, including which ingredients may be beneficial for osteoporosis and bone health and which may be a safety concern.

CL Answer
Do supplements help with Parkinson’s disease treatment or prevention?
Learn what has been shown with vitamins D and E, niacin, CoQ10, melatonin, creatine, SAMe, NAC, valerian, CoQ10, and CBD, as well as with coffee and the Mediterranean and MIND diets for treating and/or preventing Parkinson's disease.

CL Answer
What is lumbrokinase, does it have heart benefits, is it safe, and what should you look for, or avoid, in a lumbrokinase supplement?
Lumbrokinase is marketed as promoting healthy circulation and supporting heart health. Find out how and if lumbrokinase supplements actually work, possible safety concerns, and what to consider when choosing a lumbrokinase supplement.

News Release
July 31, 2019
ConsumerLab Tests Reveal Best CoQ10 and Ubiquinol Supplements
White Plains, New York, July 31, 2019 — CoQ10 is among the most popular supplements, commonly taken to offset a decline in natural levels of CoQ10 that can occur with the use of statin medications, decrease statin side effects, and increase energy.
News Release
June 25, 2019
ConsumerLab Tests Reveal Best B Vitamin Supplements -- 19% of B Vitamin Supplements Fail CL's Tests of Quality
White Plains, New York, June 25, 2019 — B vitamins and complexes are among the most popular supplements sold in the U.S. because B vitamins are essential for a wide range of functions in the body.
News Release
March 07, 2019
Best Coconut and MCT Oils Identified by ConsumerLab
White Plains, New York, March 7, 2019 — Coconut oil is often promoted as a "healthy fat" and an alternative source of energy to help with weight loss and in conditions such as Alzheimer's disease because it contains medium chain triglycerides (MCTs).
Product Review
Water Filter Pitchers Review
See the Best Pitchers For Different Filtering Needs

Product Review
Extra Virgin Olive Oil Review
Many Extra Virgin Olive Oils Don't Seem to Make the Grade

Recalls & Warnings
December 13, 2016
Case of Hemorrhagic Stroke Linked to Redline Energy Drink
A case of hemorrhagic stroke has been linked to the consumption of a single energy drink, according to a report published on October 11, 2016, in The American Journal of Emergency Medicine.
CL Answer
Pros and Cons of Stevia and Other Sugar Substitutes
Learn about the pros and cons of sugar substitutes and artificial sweeteners summarized in our table within this article.

Recalls & Warnings
August 16, 2023
FDA Warns Sellers of Homeopathic Products for Infants and Children
On August 9, 2023, the FDA issued a warning letter to ALVA-AMCO Pharmacal Companies, LLC and CalmCo LLC, previously named Ketomi LLC, following a review of the company websites, which found statements about company homeopathic products to be drug claims because they are intended to diagnose, cure, ...
Recalls & Warnings
July 24, 2023
ONO Overnight Oats Recalled Due to Allergen Risk
On July 18, 2023, ONO LLC issued a voluntary recall of the company’s ONO Vegan Blueberry Muffin Protein Overnight Oats due to undeclared milk.
Recalls & Warnings
June 14, 2023
Havasu Beet Root Powder Recalled Due to Allergen Risk
On June 12, 2023, Supplement Manufacturing Partner Inc. issued a recall of one lot of Havasu Nutrition’s Beet Root Powder + due to undeclared milk. The recall was initiated following a report of an allergic reaction from a consumer with a milk allergy.
Recalls & Warnings
June 19, 2023
A&Z Pharmaceutical Warned for Promoting Calcium & Vitamin D for Hair Loss, Osteoporosis & Cancer
On June 1, 2023, the FDA issued a warning letter to A&Z Pharmaceutical, Inc. following inspection of the company’s website and social media, which found statements about the company’s Chewable Calcium 600MG with Vitamin D for Kids in Orange Flavor products to be drug claims.
Recalls & Warnings
June 22, 2023
Nationwide Pharmaceutical’s Ferrous Sulfate Supplements Recalled
On June 22, 2023, the U.S.
Recalls & Warnings
November 30, 2023
Original The Rock Capsules Recalled Due to Undeclared Sildenafil
On October 18, 2023, Noah’s Wholesale, LLC issued a nationwide recall of one lot of the company’s Original The Rock capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
News Release
January 31, 2019
Contamination Still an Issue in Some Greens and Whole Food Products, ConsumerLab Tests Reveal
White Plains, New York, January 31, 2019 — Powders and capsules containing ingredients such as wheat grass, spirulina, chlorella, and fruits and vegetables are a popular way to get vitamins, minerals and other plant-based nutrients, but past tests by ConsumerLab have found that these products ...
Recalls & Warnings
September 03, 2019
Federal Court Shuts Down Two Supplement Companies Selling Weight, Joint Health and Other Supplements
On September 3, 2019, a U.S. District Court entered a consent decree of permanent injunction against Basic Reset and Biogenyx, two Tennessee-based companies that sell dietary supplements and other product promoted for health benefits.
Recalls & Warnings
May 09, 2022
$149 Million in Refunds from AdvoCare Settlement
On May 5, 2022, the FTC announced it will be returning more than $149 million to individuals who sold AdvoCare supplements as distributors through the company’s multi-level-marketing (MLM) plan.
News Release
March 22, 2016
Rhodiola: Does It Help With Depression and Anxiety and Which Brands Are Best? -- ConsumerLab.com Reviews the Evidence and Tests the Quality of Popular R. Rosea Supplements --
White Plains, New York, March 22, 2016 — Rhodiola (R. rosea) supplements are promoted to treat depression, anxiety, and fatigue — but do they really work? And do products on the market contain the right species, amounts, and appropriate doses of R.
News Release
January 12, 2016
Problems Found with 32% of Multivitamin/Multimineral Supplements -- ConsumerLab.com Identifies Good Choices for Men, Women, and Children
White Plains, New York, January 12, 2016 — You can't always judge a supplement by its label -- or by its price, according to a new report from ConsumerLab.com, which recently tested dozens of multivitamin/multimineral supplements.
News Release
March 05, 2015
ConsumerLab.com Shows Chinese Consumers What's Really in Foods and Supplements -- U.S. Testing Company Expands to China, Launches cn.ConsumerLab.com
White Plains, New York, March 5, 2015 — Since 1999, American consumers and doctors have turned to ConsumerLab.com (CL) to find out which nutritional products are highest in quality based independent testing. Today, CL launched a Chinese-language website cn.ConsumerLab.
Recalls & Warnings
August 03, 2023
Arsenic Poisoning in U.S. from Ayurvedic Supplements
A 75-year-old woman in California experienced arsenic poisoning after taking Ayurvedic supplements prescribed to her by a holistic energy healer, according to a report published in July in Clinical Case Reports.
Recalls & Warnings
July 12, 2023
FDA Warns Company for Promoting Kratom Products for Pain, Mood, and More
On July 3, 2023, the FDA issued a warning letter to Sunshine Trading Company, Inc.
Recalls & Warnings
November 11, 2015
Consuming a Single Energy Drink May Increase Cardiovascular Risk
A recently published study found that consumption of a single caffeinated energy drink significantly increased blood pressure and noradrenaline levels in healthy young adults. The researchers noted these changes could potentially increase the risk of cardiovascular events.
Recalls & Warnings
July 29, 2017
Increase in Calls to Poison Control Centers About Supplements
The number of calls to poison control centers in the U.S. about dietary supplement exposures increased by almost 50% between 2005 and 2012, according to a study published this week in the Journal of Medical Toxicology.
Recalls & Warnings
October 05, 2019
Advocare to Pay $150 Million to Settle Charges of Operating a Pyramid Scheme
AdvoCare International, the marketing company that sells Spark energy drink, as well as other nutrition products and supplements, has agreed to settle FTC charges that it operated an illegal pyramid scheme.
Recalls & Warnings
February 26, 2020
Company Failed to Report Adverse Events Associated With Its Nutrition Shakes
On February 12, 2020, the FDA sent a warning letter to Market America Inc for failure to submit serious adverse event reports about two of its products, as required by federal regulation. The company received two reports following serious adverse events but did not submit the proper forms:
Recalls & Warnings
February 18, 2020
Seller of CoQ10, Resveratrol and More Warned for Manufacturing Violations
On February 5, 2020, the FDA issued a warning letter to R-Garden LLC, which found the company's Vitamin O, Gamma-Zyme, L.
Recalls & Warnings
July 11, 2011
Use of "Sports Drinks" and "Energy Drinks" Discouraged by Pediatric Group
A report from the American Academy of Pediatrics outlines how sports and energy drinks are being misused and provides guidance to decrease or eliminate consumption by children and adolescents.
Recalls & Warnings
January 19, 2022
FDA Warns Seller of Carnitine and Alpha Lipoic Acid Supplements
On November 18, 2021, the FDA issued a warning letter to GeroNova Research Inc.
Recalls & Warnings
May 24, 2021
U.S. Marshals Seize $1.3 Million Worth of Kratom
U.S. Marshals have seized approximately $1.3 million worth of bulk kratom and kratom supplements manufactured by Atofil, LLC, a subsidiary of Premier Manufacturing Products, according to the FDA.
News Release
December 09, 2014
Round-up of Recent Product Tests by ConsumerLab.com -- Results for B Vitamin Supplements & Energy Drinks, Garlic, NAC (N-acetyl cysteine), Iron and Omega-7 Fatty Acids
White Plains, New York, December 9, 2014 — In recent months, ConsumerLab.com has published test results for a variety of popular supplements, including B vitamins supplements and energy drinks, NAC (N-acetyl cysteine), iron and garlic. In addition, ConsumerLab.
News Release
October 08, 2013
ConsumerLab.com Reports Problems and “Top Picks” Among Bars for Energy, Fiber, Protein, Meal-Replacement, and Whole Food
WHITE PLAINS, NEW YORK — October 8, 2013 — Which nutrition bars provide the best combination of nutrition and taste, and are accurately labeled? ConsumerLab.com recently set out to answer this question by purchasing popular bars in the U.S.
News Release
July 08, 2013
Contamination a Common Problem in "Greens" and "Whole Foods" Products According to ConsumerLab.com
White Plains, New York — July 8, 2013 — "Greens" and "whole foods" powders and pills, made from wheat grass, alfalfa, kelp, spirulina, leafy vegetables and other chlorophyll-containing ingredients, as well as fruits, were recently tested by ConsumerLab.
News Release
June 11, 2013
31% of Protein Powders and Drinks Fail Tests by ConsumerLab.com
White Plains, New York — June 11, 2013 — What's really in a scoop of "protein powder"? sIn some products, a lot less protein or a lot more carbohydrates than you would expect, and perhaps even a serving of something you don't want -- lead. That's according to recent tests by ConsumerLab.
News Release
September 27, 2012
ConsumerLab.com Reveals How Much Caffeine is in Energy Drinks -- Also Finds Some Drinks and Dietary Supplements Don't Contain Claimed Amounts of B Vitamins
White Plains, New York — September 27, 2012 — Energy shots and drinks are promoted to keep you alert and energized, attributing their effects to special formulas often "packed with B vitamins and nutrients to make it last," as a commercial for 5-hour Energy proclaims.
Recalls & Warnings
August 16, 2023
FDA Warns Hekma Center, LLC for Promoting Products to Treat Anemia, Diabetes, Depression, & More
On June 2, 2023, the FDA issued a warning letter to Hekma Center, LLC following review of the company’s website and social media, which found statements about the company’s Natural Supplements for Anemia, Lymf (Galium aparine), Natural Supplements for Cardiomyopathy, Magic1 (Moringa ...
News Release
February 05, 2012
Fish oil and multivitamins most popular supplements in ConsumerLab.com survey -- Internet most popular place to shop
WHITE PLAINS, NEW YORK — FEBRUARY 5, 2012 — A survey of over 10,000 savvy consumers of supplements shows the most popular supplements to be fish oil, multivitamins, vitamin D, calcium and CoQ10, in that order.
Recalls & Warnings
June 05, 2020
Seller of CBD, Sleep Aids, and Cold & Flu Products Warned for Manufacturing Violations
On April 28, 2020, the FDA issued a warning letter to The Dragontree Apothecary LLC, which found the company's Sleep Support, Anxiety Relief, Cold & Flu Relief, and Inflammation Relief to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
June 02, 2020
Sundial Herbal Ordered to Halt Distribution of Products
On June 1, 2020, a New York federal judge filed an order against two individuals doing business as Sundial Herbal Products to stop the company from distributing its products, which were found to be misbranded and promoted as drugs.
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
January 31, 2021
Protein and Fiber Bars Recalled Due to Allergen Risk
On January 28, 2021, think! and Interpac Technologies, Inc. issued a voluntary recall of think! Protein + Fiber Oatmeal: Farmer's Market Berry Crumble due to the potential presence of tree nuts, including almonds and pecans.
Recalls & Warnings
November 21, 2012
Distributor Warns of Counterfeit Liquid Energy Shots
On November 19, 2012, Living Essentials LLC announced the company discovered and halted the production of counterfeit versions of 5-hour ENERGY® liquid energy shots. The company filed suits against the counterfeiters in U.S.
Recalls & Warnings
August 31, 2012
Energy, Fat Loss Supplement Recalled For Ephedrine Alkaloids
On August 28, 2012, dietary supplement re-sale distributor Brand New Energy (BNE) issued a recall of EphBurn 25 after FDA testing found the product to contain ephedrine alkaloids.
News Release
October 26, 2010
ConsumerLab.com reports deficiencies in some B-complex supplements and more caffeine than expected in "shot-sized" B vitamin "energy" drinks
White Plains, New York — October 26, 2010 — Tests of B vitamin supplements, including B-complexes and shot-sized energy drinks, revealed problems with the quality of 4 out of 18 products selected for review by independent testing organization ConsumerLab.com.
Recalls & Warnings
December 07, 2016
Chocolate Hazelnut Butter CLIF Nut Butter Filled Energy Bars Recalled
On November 18, 2016, Clif Bar & Company issued a recall of one production run of Chocolate Hazelnut Butter CLIF Nut Butter Filled energy bars due to the presence of small plastic pieces found in a limited number of bars.
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
March 26, 2021
Nine Banned Stimulants Found in Workout, Weight Loss Supplements
Recent analysis of 17 brands of sports/energy and weight loss supplements sold in the U.S. found nine prohibited stimulants formulated into eight different combinations, and none of these combinations have been studied in people.
Recalls & Warnings
March 18, 2021
Don't Drink Real Water Alkaline Water, FDA Warns After Reports of Liver Illness
On March 16, 2021, the FDA warned consumers not to drink or use Real Water bottled alkaline water while it investigates reports of hepatitis associated with the product.
Recalls & Warnings
May 23, 2020
FTC Warns 50 More Companies for Coronavirus Claims
On May 21, 2020, the FTC announced that it sent warning letters to 50 companies for selling products such as herbal products, immune system boosters, and vitamin C, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 03, 2019
Vinpocetine May Cause Miscarriage or Fetal Harm
Women who are pregnant or who could become pregnant should not take Vinpocetine because it may cause miscarriage or harm fetal development, the FDA warned in a news release today.
News Release
August 16, 2010
Problems persist with ginseng supplements -- Review by ConsumerLab.com finds 45% of products don't provide full amount of ingredient or are contaminated
WHITE PLAINS, NEW YORK — August 16, 2010 — ConsumerLab.com reported today that five out of eleven ginseng supplements recently selected for testing contained less ginseng than expected from their labels or were contaminated with lead and/or pesticides.
News Release
May 05, 2010
Tests of protein powders and drinks show some lead contamination but no melamine -- New report covers whey, soy, and rice protein supplements for body building, endurance/recovery, meal-replacment and dieting
WHITE PLAINS, NEW YORK — MAY 5, 2010 — New tests by ConsumerLab.com found most protein powders, shakes and drinks to meet their nutrient claims, but two products were contaminated with lead (6 to 18 mcg per day) and a third product contained four extra grams of sugar.
Recalls & Warnings
February 15, 2013
Energy Drink Recalled Due To Bacterial Contamination
On December 21, 2012, NBTY issued a voluntary recall of the energy supplement MET-Rx Extreme Thermo Rage Watermelon 8 fl. oz. (Manufactured by MET-Rx Nutrition, Inc.) due to bacterial contamination.
Recalls & Warnings
May 10, 2007
FDA Warns of Two Supplements Containing Pharmaceutical-like Compounds
On May 10, 2007, the FDA advised consumers not to purchase or use "True Man" or "Energy Max" products promoted and sold as dietary supplements throughout the United States.
Recalls & Warnings
June 25, 2019
Kratom Is Dangerous and Not Proven to Treat Addiction, FDA Warns Marketers
On June 25, 2019, the FDA issued warning letter to two marketers and distributors of kratom products, listed below, for illegally selling unapproved, misbranded kratom-containing drug products with unproven claims about their ability to treat or cure opioid addiction and withdrawal symptoms.
Recalls & Warnings
July 06, 2012
Centrum Multivitamins: Breast and Colon Health Claims Pulled
On July 5, 2012, Pfizer Consumer Healthcare announced it will withdraw breast and colon health claims from its Centrum multivitamin advertising and labels, and will revise health and energy claims made on other Centrum products.
Recalls & Warnings
June 07, 2013
Nutrition Bars Recalled Due To Undeclared Milk Allergen
On June 6, 2013, Sequel Naturals Ltd. issued a recall of Vega sports, nutrition and energy bars because they contain undeclared milk. Consumption of these bars can cause serious or life-threatening reactions in people with an allergy to milk.
Recalls & Warnings
April 18, 2013
Cardio, Energy and Sexual Enhancement Supplement Distributor Warned For Manufacturing Violations and Drug Claims
On December 21, 2012, the FDA issued a warning letter to ForMor Inc, dba ForMor International, following a facility inspection which found the company's Cardio Cocktail and Argenix dietary supplements to be adulterated because because they were prepared, packed, or held under conditions that do ...
Recalls & Warnings
September 30, 2015
Weight and Energy Supplement Contains Synthetic "Amphetamine"
On September 21, 2015, the FDA issued a warning letter to TruVision Health LLC. because the company's supplement tru Weight & Energy lists a synthetic, amphetamine-like compound, AMP (also called 1,3-dimethylbutylamine or DMBA) on product labels.
Recalls & Warnings
June 03, 2015
Maker of B-12 Energy Supplement Warned for Manufacturing Violations
On May 15, 2015, the FDA issued a warning letter to LiquidCapsule Manufacturing, LLC.
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
November 25, 2007
Recall of Men's "Sexual Energy" Supplement Expanded to Include "Energy Max" Product
On November 16, 2007, American True Man Health Incorporated announced that it is expanding it's voluntary recall of the Company's dietary supplement product sold under the name and identified as True Man's Sexual Energy Nutriment Men's Formula with the expiration date up to and including December ...
Recalls & Warnings
July 10, 2018
Blissful Remedies Kratom Recalled Due to Salmonella Risk
On June 30, 2018, Blissful Remedies issued a recall of three kratom products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
June 19, 2018
FDA Warns Seller of Weight Control Patches
On June 6, 2018, the FDA issued a warning letter to Health Management Group, Inc.
Recalls & Warnings
May 29, 2018
FDA Warns Seller of "Aromatase Inhibitor" Supplement
On May 18, 2018, the FDA issued a warning letter to Performance Nutrition Formulators LLC d.b.a. VMI Sports because its product Arimistane contains Androsta-3,5-Diene-7,17-Dione, an ingredient that the FDA considers to be a new drug and not a dietary supplement ingredient.
Recalls & Warnings
April 16, 2019
DMHA and Phenibut Are Not Permitted in Dietary Supplements, Warns FDA
On April 16, 2019, the FDA announced it has issued 11 warning letters to companies whose dietary supplement products contain the drugs DMHA or phenibut, and therefore are in violation of the law.
Recalls & Warnings
November 27, 2018
High Levels of Lead Found in Kratom Products
Some kratom products contain levels of lead and nickel that are not considered safe for human consumption, according to tests conducted by the FDA.
Recalls & Warnings
September 19, 2018
FDA Warns Seller of Shilajit
On September 6, 2018, the FDA issued a warning letter to Adaptive Energy LLC, following an inspection of the company's website, which found statements made about its shilajit product, Purblack were found to be drug claims.
Recalls & Warnings
September 09, 2018
Higenamine -- A Potentially Dangerous Stimulant -- Found in Some Supplements
A recent study found concerningly high doses of the stimulant higenamine in some weight loss, energy and sports supplements sold in the U.S. Higenamine is a naturally-occurring stimulant which is permitted to be sold as a dietary supplement ingredient in the U.S.
News Release
August 12, 2008
ConsumerLab.com finds improvements in labeling of nutrition bars but potential pitfalls exist -- New report compares 20 bars, including those for protein, fiber, energy and whole food
WHITE PLAINS, NY — August 12, 2008 — Labeling on nutrition bars has become more accurate, according to a new report by ConsumerLab.com.
News Release
August 20, 2007
ConsumerLab tests products in booming nutrition drink market — most deliver what's promised but extra cholesterol found in some — New report covers drinks and powders for endurance/recovery, body building, meal-replacement and dieting
WHITE PLAINS, NEW YORK — AUGUST 20, 2007 — A new report from ConsumerLab.com finds most nutrition powders, shakes and drinks meet their nutrient claims, but three products — two sports drinks and a meal supplement — contained more cholesterol than claimed.
News Release
May 24, 2006
Problems persist with ginseng supplements — ConsumerLab.com finds nearly half of products lack ingredient or contaminated
WHITE PLAINS, NEW YORK — May 24, 2006 — ConsumerLab.com has released test results for dietary supplements made with ginseng, a popular herb promoted for vitality and the treatment or prevention of a range of medical conditions.
News Release
June 20, 2005
Quality problems widespread among ginseng products sold in Japan. — Nearly two-thirds of products fail ConsumerLab.com testing due to pesticide contamination and missing or substandard ingredients
WHITE PLAINS, NY — JUNE 20, 2005 — Among 14 brands of ginseng dietary supplements recently purchased in Japan and tested by ConsumerLab.com, only 5 products passed independent testing by ConsumerLab.com.
News Release
February 08, 2005
ConsumerLab.com reports on nutrition bars — Highlights major differences, inaccuracies, and urges consumers to "know your bar"
WHITE PLAINS, NY — February 8, 2005 — ConsumerLab.com reported test results today for 34 nutrition bars, including those designed for high protein, low-carb/diet, energy, or meal-replacement.
Recalls & Warnings
December 06, 2013
Weight Loss Supplement Claims Challenged
On November 27, 2013, the National Advertising Division (NAD) recommended HealthyLife Sciences, LLC, modify or discontinue the use of certain claims made about the company's weight loss supplement Healthe Trim.
Recalls & Warnings
January 28, 2014
Emergen-C Settles False Advertising Lawsuit
Alacer Corp., marketers of Emergen-C, has agreed to pay $6.45 million to settle a class action lawsuit which charged the company made false claims that the vitamin C supplement could boost immunity, energy and metabolism.
Recalls & Warnings
November 20, 2013
Another Meth-Like Compound Discovered In Weight Loss Supplements
A "non-natural" amphetamine-like compound, identified as beta-methylphenethylamine, was discovered in nine supplements recently tested by FDA scientists.
Recalls & Warnings
June 14, 2013
Protein And Nutrition Bars Recalled Due To Undeclared Milk Allergen
On June 13, 2013, the U.S. FDA alerted consumers that Sequel Naturals Ltd., dba Vega, issued a voluntary recall of a variety of Vega One and Vega Sport protein bars because the bars contain undeclared milk.
Recalls & Warnings
August 29, 2012
Herbal Supplement Company Warned For Medical Claims
On August 2, 2012, the FDA issued a warning to HSAC Enterprises, Inc. dba Kare-N-Herbs subsequent to a facility inspection and website review in May 2012 which found statements made about Kold Kare, Energy Kare and Tranquility Kare dietary supplements to constitute drug claims.
Recalls & Warnings
October 15, 2012
Recall of Nutrition Bars Containing Peanut Butter Due To Salmonella Risk
On October 11 and 12, 2012, four brands of nutrition bars were voluntarily recalled due to possible salmonella contamination of the peanut butter used in each of these bars. The peanut butter was produced by Sunland Inc., and is part of a larger recall of Sunland's peanut butter and peanut products.
Recalls & Warnings
March 19, 2009
QVC Settles Charges of False Claims for Supplements
On March 19, 2009, the Federal Trade Commission (FTC) announced that QVC, Inc., a TV home shopping channel and one of the world’s largest multimedia retailers, has agreed to pay $7.
Recalls & Warnings
July 19, 2014
Powdered Caffeine Can Be Lethal, FDA Warns
On July 18, 2014, the FDA warned consumers to avoid powdered pure caffeine sold in bulk bags over the internet.
Recalls & Warnings
December 30, 2014
Maker of Aloe, Weight Loss Supplements and More Warned for Manufacturing Violations, Drug Claims
On December 17, 2014, the FDA issued a warning letter to Dandy Day Corporation, following a facility inspection which found the company's products, including Aloe Pearl, Alfalo, Aloespring, Crave Away, Original Supreme Energy, Ener "G," Can G, Flora G, Flora G Plus, and Garolic, to be adulterated ...
Recalls & Warnings
September 27, 2016
Seller of B Vitamins, Omega-3s and More Warned for Manufacturing Violations, Drug Claims
On September 15, 2016, the FDA issued a warning letter to Positive Power Nutrition, following a facility inspection which found the company's products, including High Energy C-Complex, Positive Vitality, Positive Performance, Positive CardioGuard, B-Complex 100, Positive Essentials, Positive ...
Recalls & Warnings
June 02, 2016
Clif Bars Recalled Due to Listeria Risk
On June 2, 2016, Clif Bar & Company issued a recall of certain energy and trail mix bars because they contain sunflower kernels which have the potential to be contaminated with Listeria monocytogenes.
Recalls & Warnings
February 24, 2015
Seller of Antioxidant Water, Energy Drops Warned for Manufacturing Violations and Drug Claims
On February 18, 2015, the FDA issued a warning letter to Better Health Lab, Inc.
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
August 16, 2018
Kratom Recalled Due to Salmonella Risk
On August 14, 2018, Zakah Life, LLC issued a recall of Super Green Maeng Da Premium Kratom powder, Powerful Red Vein Bali Premium Kratom powder, Super Green Maeng Da Premium Kratom capsules, and Powerful Red Vein Bali Premium Kratom capsules because laboratory testing revealed the presence of ...
Recalls & Warnings
January 23, 2013
USPLabs Settles Class Action Lawsuit Over Controversial DMAA Ingredient
On December 18, 2012, a settlement was approved in the class action lawsuit against USPLabs, which alleged the company's use of the ingredient DMAA (1,3-dimethylamine) in the supplements OxyELITEPRO and Jacked3d was not safe or legal.
Recalls & Warnings
March 09, 2012
Maker of Inhaled Caffeine Product Gets Warning
The U.S. FDA issued a Warning Letter to Breathable Foods, Inc. (dated March 5, 2012) advising that their product AeroShot, which is promoted for "breathable energy," is falsely labeled (misbranded).
Recalls & Warnings
October 16, 2013
Methamphetamine-Like Compound Found In Popular Workout Supplement
A study published on October 14, 2013 (Cohen, Drug Test Analysis 2013) reports that the popular pre-workout energy supplement Craze (Driven Sports) has been found to contain the banned methamphetamine — like compound, N, á-diethylphenylethylamine.
Recalls & Warnings
September 05, 2013
Seller of Herbal Supplements and "Tonics" Warned For Manufacturing Violations and Drug Claims
On May 24, 2013, the FDA issued a warning letter to Sundial Herbal Products following a facility inspection which found the company's products, including Woman Back Tonic, Koromantee, Wood and Root Tonic, Arthritis, Asthma, Diabetics, Sundial Ashanti Weight Loss Energy Lifter, Appetite Suppresser, ...
Recalls & Warnings
January 23, 2015
Maker of Vitamin Drinks Warned for Manufacturing Violations
On January 7, 2015, the FDA issued a warning letter to NYSW Beverage Brands, Inc.
Recalls & Warnings
October 08, 2014
Consumers Who Have Purchased Red Bull May Qualify for Cash Payment or Free Red Bull Products
Consumers who purchased Red Bull products in the United States between January 1, 2002 and October 3, 2014 may be entitled to a $10 cash payment or two free Red Bull products, according to a proposed settlement of two class action lawsuits.
Recalls & Warnings
March 17, 2015
Seller of Tea Warned for Drug Claims
On March 4, 2015, the FDA issued a warning letter to Four Elements Organic Herbals, LLC because statements made about HERBAL TEA Power, Energy & Stamina, ORGANIC HERBAL TEA Tulsi TelepaTea, ORGANIC HERBAL TEA To Your Health, Hawthorn Fresh Herb Extract, Elderberry Fresh Herb ...
Recalls & Warnings
October 17, 2015
23,000 ER Visits Linked to Supplements: Palpitations, Swallowing Problems, Allergies Common
Approximately 23,000 emergency room visits per year are attributed to adverse events linked to the use dietary supplements, according to a large, long-term study by the FDA and the Centers for Disease Control and Prevention (CDC).
Recalls & Warnings
June 15, 2016
More Nutrition Bars Recalled Due to Listeria Risk
The following nutrition bars are being recalled because they contain sunflower kernels or may have come in contact with equipment processing sunflower kernels which have the potential to be contaminated with Listeria monocytogenes (use the links below for affected lot numbers, UPC, and/or ...
Recalls & Warnings
March 15, 2016
FDA Warns Sellers of Weight and Workout Supplements Containing Acacia Rigidula
On March 7, 2016, the FDA issued warning letters to five sellers of supplements which were labeled as containing Acacia rigidula, an ingredient which is not permitted in dietary supplements.
Recalls & Warnings
May 10, 2016
Multis and Energy Mixes Recalled
On May 9, 2016, Let's Talk Health, Inc. issued a recall of the following supplements because they contain the undeclared allergens soy and milk:
Recalls & Warnings
April 10, 2021
HI-TECH Workout Supplements Recalled Due to Undeclared Allergens
On April 3, 2021, HI-TECH Pharmaceuticals issued a recall of all lots of APS Nutrition Isomorph 28 in a 2lb jug because they contain undeclared milk, wheat, and soy.
Recalls & Warnings
July 20, 2017
Jail Time for Seller of Spiked Supplements
On July 18, 2017, Derek Vest, the former president of Gentech Pharmaceutical located in Fort Meyers, Florida, was sentenced to 18 months in prison for introducing misbranded food into interstate commerce.
Recalls & Warnings
July 08, 2017
CDC Warns Against Placenta Pills After Infant Becomes Sick
An infant in Oregon whose mother began taking capsules of dried placenta three days after giving birth was diagnosed with late-onset group B Streptococcus agalactiae (GBS) bacteremia, a strep infection which was linked to the placenta pill, according to a recently published report from the ...
Recalls & Warnings
May 26, 2017
"Allergy" Supplement Containing Ephedra Recalled
On February 7, 2017, MusclMasster, LLC (DBA The Green Herb) of Wheat Ridge, CO issued a recall of all bottles of Al-Er-G Capsules , a product promoted to help allergies, because they contain ephedra.
Recalls & Warnings
May 26, 2017
Herbal "Sexual Enhancement Coffee" Recalled, One Death Reported
On May 25, 2017, Caverflo.com issued a recall of Caverflo Natural Herbal Coffee, an "herbal" instant coffee promoted for sexual enhancement, because it was found to contain the drugs sildenafil and tadalafil.
Recalls & Warnings
April 21, 2018
NxtGen Botanicals Maeng Da Kratom Recalled
On April 18, 2018, NGB Corp. of West Jordan, Utah issued a recall of NxtGen Botanicals Maeng Da Kratom labeled bottles of encapsulated product because they have the potential to be contaminated with Salmonella. To date, one illness has been reported to the FDA.
Recalls & Warnings
April 09, 2018
Kratom Powders and Capsules Recalled Due to Salmonella Risk
On April 5, 2018, Club 13 issued a recall of certain kratom powders and capsules because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
February 06, 2018
FDA Links 44 Deaths to Kratom
On February 6, 2018, FDA announced it is now aware of 44 deaths associated with the use of kratom, an herb often promoted for pain relief, energy and for treating opioid withdrawal symptoms. This is an increase from the 36 deaths associated with kratom that the agency reported in November 2017.
Recalls & Warnings
November 14, 2017
36 Deaths Associated With the Use of Kratom Products
On November 14, 2017, FDA Commissioner Scott Gottlieb, M.D. issued a statement on the dangers of using products containing kratom, an herb often promoted for pain relief, energy and for treating opioid withdrawal symptoms.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
October 04, 2017
Police Officer Dies From Kratom Overdose, Herb Currently Legal in Most States
A police officer who worked in narcotics in Tupper Lake, New York, died this summer of an overdose of the herb kratom, according to a report from ABC News.
Recalls & Warnings
September 26, 2017
FDA Warns Seller of DHEA, Creatine, Chromium & More for Manufacturing Violations, Drug Claims
On August 25, 2017, the FDA issued a warning letter to Mega-Pro Nutrition, Inc., following inspections of the facility and website www.mega-pro.
Recalls & Warnings
August 29, 2017
FDA Warns Seller of Supplements for Allergies, Joint Pain, Bone Health, and More
On August 16, 2017, the FDA issued a warning letter to Total Nutrition, Inc.
Recalls & Warnings
August 20, 2013
Weight Loss Supplement Formulas For Men and Women Recalled Due To Undeclared Drugs
On August 16, 2013, Herbal Give Care LLC issued a voluntary recall of all lots of weight loss dietary supplements Esbelder man, Esbelder fem and Esbelder siloutte because they were found to contain the undeclared drugs sibutramine, N-desmethylsibutramine, and N-di-desmethylsibutramine.
Recalls & Warnings
August 01, 2013
Fat Loss and Muscle Enhancement Supplements Found To Contain DMAA
On June 17, 2013, the FDA issued a warning letter to Formulife, Inc., dba Purus Labs, Inc., following a facility inspection which found the dietary supplements Fat Smack XR Thermolipolytic, Muscle Marinade Fresh Fruit and Muscle Marinade Cherry Limeade to contain DMAA.
Recalls & Warnings
July 26, 2013
Safety of Certain Weight Loss and Bodybuilding Supplements Called Into Question
On July 25, 2013, USA Today reported that several supplements, including weight loss, body building and sports nutrition supplements, have come under scrutiny after being found to contain illegal ingredients, or were associated with failed drug tests and adverse events.
Recalls & Warnings
February 13, 2013
Manufacturer of Heart, Multivitamin, Cranberry, and Longevity Supplements Warned For Adulteration and Drug Claims
On January 29, 2013, the FDA issued a warning letter to M.D.R. Fitness Corp. following a facility inspection which found violations of good manufacturing practices which cause the company's products to be adulterated.
Recalls & Warnings
November 01, 2016
FDA Warns Consumers Not to Use Twelve Energy and Sexual Enhancement Supplements
The FDA recently warned consumers not to buy or use the following "energy" and sexual enhancement supplements, which are sold on various websites and in some retail stores, because they were found to contain undeclared drugs:
Recalls & Warnings
March 19, 2014
Maker of Herbal Capsules and Extracts Warned for Manufacturing Violations
On September 19, 2013, the FDA issued a warning letter to Herbalist and Alchemist, Inc.
Recalls & Warnings
June 24, 2014
CELLFOOD Recalled in Canada
On June 23, 2014, Health Canada recalled CELLFOOD (Nu Science), a supplement promoted for improved energy, endurance and natural health, because it was found to be an unauthorized health product.
Recalls & Warnings
April 03, 2014
Maker of Green Coffee Bean Extract and Weight Loss Supplement Warned for Manufacturing Violations
On March 13, 2014, the FDA issued a warning letter to Libi Labs, Inc.
Recalls & Warnings
March 03, 2012
FDA Warns Vitaganic of Manufacturing Violations
On February 8, 2012, the U.S. FDA sent a Warning Letter to Vitaganic, Inc. regarding violations of Current Good Manufacturing Practic (CGMP) regulations for dietary supplements found during an inspection of it manufacturing facility in Sunnyvale, California in October 2011.
Recalls & Warnings
January 04, 2012
Seller of "HCG Drops" for Weight Loss Warned by FDA and FTC of Violations
On December 21, 2011, the U.S. FDA and FTC sent a joint Warning Letter to hCG Drops LLC regarding its marketing of Slim Diet Drops. According to the letter, the product is considered an unapproved drug because it is promoted for drug-like uses on the company's website, including the following:
Recalls & Warnings
May 07, 2012
FDA Warns Doctor Promoting Own Supplements Online as Treatments
On April 18, 2012 the U.S. Food and Drug Administration (FDA) sent a Warning Letter to Jacob Teitelbaum, M.D. of Fatigued to Fantastic, LLC regarding the promotion that company's products on its website www.endfatigue.com.
Recalls & Warnings
October 25, 2013
Supplement Company Warned For Numerous Manufacturing Violations
On August 2, 2013, the FDA issued a warning letter to DNE Nutraceuticals, Inc., following a facility inspection which found the company's products to be adulterated because they were packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
December 19, 2013
Maker of Joint Health Supplements Warned for Manufacturing Violations
On December 4, 2013, the FDA issued a warning letter to PurQuality, LLC.
Recalls & Warnings
April 04, 2012
Maker of Protein Drinks and Supplements Warned of Manufacturing Violations
The FDA published a Warning Letter to Protica, Inc., a maker of protein drinks and supplements, regarding violations of manufacturing regulations discovered during inspection of its facility in Whitehall, PA. Affected products include the foods Amped Up-2oz, Fireball-2oz, Healthy Shot-2.
Recalls & Warnings
June 23, 2018
Gaia Kratom Recalled Due to Salmonella Risk
On June 21, 2018, Gaia Ethnobotanical, LLC issued a recall of 27 kratom products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
February 22, 2018
Kratom Supplements Recalled and Destroyed
On February 21, 2018, the FDA announced the voluntary destruction and recall of Botany Bay, Enhance Your Life and Divinity kratom supplements. All of the products were manufactured and distributed nationwide by Divinity Products Distribution, located in Grain Valley, Missouri.
Recalls & Warnings
February 20, 2018
Salmonella Outbreak Linked to Kratom Powders, Pills and Teas
On February 20, 2018, the Center for Disease Control (CDC) warned consumers not to use kratom in any form because it could be contaminated with Salmonella. The CDC is currently investigating an outbreak of illness caused by Salmonella infection associated with the use of kratom.
Recalls & Warnings
April 03, 2018
FDA Issues Mandatory Recall of Salmonella-Contaminated Kratom Products
On April 2, 2018, the FDA issued a mandatory recall of all food products containing powdered kratom manufactured, processed, packed, or held by Triangle Pharmanaturals LLC because several of company's products were found to be contaminated with Salmonella.
Recalls & Warnings
April 02, 2018
NutriZone Kratom Supplements Recalled Due to Salmonella Risk
On March 10, 2018, NutriZone, LLC issued a recall of certain kratom-containing powder products because they have the potential to be contaminated with Salmonella.
News Release
March 02, 2004
ConsumerLab.com survey finds most consumers highly satisfied with supplements; Ratings vary by brand
WHITE PLAINS, NY — March 2, 2004 — ConsumerLab.com announced today that 78% of consumers recently surveyed report being "extremely" or "very" satisfied with the supplement brands they used. Ratings varied by brand. The highest rating was for Nutrilite (96%), a direct sales brand.
Recalls & Warnings
March 17, 2004
Marketers of “Focus Factor” and “V-Factor” Fined for Advertising Claims
On March 17 , 2004 the Federal Trade Commission (FTC) reported that the marketers of “Focus Factor,” a dietary supplement that purports to improve concentration, and “V-Factor,” a supplement that purports to enhance sexual performance, have agreed to settle charges that they made numerous ...
Recalls & Warnings
January 11, 2002
Canada Requests Recall of Certain Ephedra/Ephedrine Products
OTTAWA - January 10, 2002 - Health Canada is requesting a recall from the market of certain products containing Ephedra/ephedrine after a risk assessment concluded that these products pose a serious risk to health.
Recalls & Warnings
March 13, 2020
FDA Finds Problems at 52% of Supplement Manufacturing Sites in U.S. and 42% Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2019 (October 1, 2018 - September 30, 2019) of 598 dietary supplement manufacturing facilities in the U.S.
Recalls & Warnings
January 31, 2002
Recall Update for Certain Metabolife Bars - Excessive Vitamin A
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its January 30, 2002 Enforcement Report.
Recalls & Warnings
January 24, 2009
Many Brands of Nutrition Bars Recalled Due to Potential Salmonella Contamination
Due to salmonella contamination in peanut butter produced by Peanut Corporation of America, manufacturers of nutrition and snack bars made with this material have recently issued voluntary recalls.
Recalls & Warnings
October 27, 2013
Ingredient In Workout Supplements May Be Unsafe, FDA Warns
On October 11, 2013, the FDA directed USP Labs, LLC to cease distribution of workout supplement OxyElite Pro and and muscle-building supplement VERSA-1, which are labeled as containing the ingredient aegeline, or N-[2-hydroxy-2(4-methoxyphenyl) ethyl]-3-phenyl-2-propenamide.
Recalls & Warnings
September 27, 2013
Company Warned For Distributing Weight Loss Supplement Containing DMAA, Drug Claims
On September 6, 2013, the FDA issued a warning letter to Pure Energy Products, Inc., following a facility inspection which found that the company distributes a weight loss supplement, called obestrim, which contains dimethylamylamine, or DMAA.
Recalls & Warnings
April 12, 2013
FDA Warns Consumers About The Dangers Of DMAA
On April 11, 2013, the FDA warned consumers about the dangers of dietary supplements containing the ingredient 1,3-dimethylamylamine (DMAA).
Recalls & Warnings
May 23, 2013
Maker of Liver Detox and Insulin Supplements Warned For Drug Claims
On April 24, 2013, the FDA issued a warning letter to Glucorell, Inc.
Recalls & Warnings
May 17, 2013
Hoodia, Sexual Enhancement Supplements and More Found To Be Adulterated, Misbranded
On March 01, 2013, the FDA issued a warning letter to Desert Rose Manufacturing, Inc.
Recalls & Warnings
April 16, 2012
Protein Supplement Maker Fails FDA Audit -- Many Products Affected
The FDA sent a Warning Letter (dated April 2, 2012) to Theta Brothers Sports Nutrition, Inc. regarding numerous violations of good manufacturing practices at its Lakewood, New Jersey facility.
Recalls & Warnings
February 20, 2013
FDA Warns USPLabs For Adulteration and Drug Claims
On December 4, 2012 the FDA issued a warning letter to USPLabs, LLC following facility inspections which found the company's products, including dietary supplements Jacked3d, OxyElite Pro, Prime, and Super Cissus, to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
January 24, 2013
"Thermo Stimulating" Supplement Recalled -- Contained DMAA
On January 23, 2013, Health Canada advised consumers not to use certain batches of Muscletech Hydroxystim capsules which were found to contain DMAA (1,3-dimethylamylamine) and have been recalled by their distributor.
Recalls & Warnings
December 21, 2012
Weight Loss Supplement Containing Undeclared Drug Recalled
On December 19, 2012, P&J Trading issued a voluntary recall of SLIMDIA REVOLUTION after FDA testing found the dietary supplement contains undeclared sibutramine.
Recalls & Warnings
September 18, 2012
Men’s Sexual Enhancement Supplement Containing Prescription Drug Recalled
On September 12, 2012, Body Basics Inc. issued a voluntary nationwide recall of ACTRA-Sx 500 Dietary Supplement Capsules after independent laboratory testing confirmed the supplement contains the prescription drug sildenafil citrate.
Recalls & Warnings
March 21, 2014
Products Containing Kratom Recalled Following FDA Import Alert
On March 14, 2014, SNI National issued a voluntary recall of all kratom supplements, including kratom XL 4 Pack, Maeng Da Kratom 10 Pack, Max Kratom 20 Pack, and Bali Kratom 40 pack, following an FDA-issued import alert on all products containing this ingredient.
Recalls & Warnings
February 13, 2014
Maker of Cholesterol and Workout Supplements Warned For Manufacturing Violations, Drug Claims and Unapproved Ingredient
On January 31, 2014, the FDA issued a warning letter to Exclusive Supplements Inc.
Recalls & Warnings
September 30, 2014
U.S. Marshals Seize $5 Million Worth of Kratom
On September 25, 2014, U.S. Marshals seized more than 25,000 pounds of raw kratom valued at over $5 million from Rosefield Management, Inc. in Van Nuys, California. The action was taken at the request of the FDA.
Recalls & Warnings
February 17, 2015
Seller of Muscle Supplements Containing Synthetic Steroids Warned
On February 9, 2015, the FDA issued a warning letter to A2Z Industries, LLC, following a facility inspection which found the company's muscle enhancing supplements, including HaloV and EPI2A3A to be labeled as containing synthetic steroids.
Recalls & Warnings
February 16, 2016
Work Out and Weight Supplements Contain Synthetic Amphetamine-Like Compound
On February 3, 2016, the FDA issued a warning letter to ATS Labs, LLC because labels for the company's work out and weight loss supplements CFI, Weapon-X Pre-Workout Extreme, and Lady Lean list a synthetic, amphetamine-like compound, 4-amino-2-methylpentane citrate (also called ...
Recalls & Warnings
January 12, 2016
Over $400,000 Worth of Kratom Supplements Seized by U.S. Marshals
On January 6, 2016, U.S. Marshals seized nearly 90,000 bottles of dietary supplements labeled as containing kratom (brand name RelaKzpro) valued at over $400,000, from Dordoniz Natural Products LLC in South Beloit, Illinois. The action was taken at the request of the FDA.
Recalls & Warnings
October 14, 2015
No Vinpocetine in Some Vinpocetine Supplements; Picamilon Labels Not Accurate
A recently published study of 23 supplements listing vinpocetine as an ingredient found six did not contain any vinpocetine. Among those that did contain vinpocetine, most did not list amounts on the label, but amounts found in suggested daily servings varied by almost 100-fold, from 0.
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
April 29, 2015
FDA Targets Weight Loss and Workout Supplements Listing Synthetic Stimulant DMBA
On April 24, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called 1,3-dimethylbutylamine (DMBA) on product labels.
Recalls & Warnings
April 08, 2015
Does Your Weight Loss or Sports Supplement Contain Synthetic Amphetamine?
A study published on April 7, 2015, reported that among 21 weight loss, sports and cognitive enhancement supplements labeled as containing the plant extract Acacia rigidula, more than half were found to contain a synthetic, amphetamine-like compound called beta- methylphenylethylamine ...
Recalls & Warnings
September 26, 2015
Seller of Muscle Supplements and More Warned for Manufacturing Violations
On August 14, 2015, the FDA issued a warning letter to Chaotic Labz, Inc.
Recalls & Warnings
September 05, 2015
Maker of Multivitamin and Fish Oil Warned for Manufacturing Violations
On July 22, 2015, the FDA issued a warning letter to Westar Nutritional Corp. dba Viva Life Science, Inc.
Recalls & Warnings
July 02, 2015
Maker of Arthritis Supplement Warned for Manufacturing Violations, Drug Claims
On June 17, 2015, the FDA issued a warning letter to Desert Stream, Inc.
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
September 02, 2016
Kratom To Be Classified a Schedule I Controlled Substance
On August 30, 2016, the U.S. Drug Enforcement Agency (DEA) announced its intent to classify the active compounds (mitragynine and 7-hydroxymitragynine) in kratom as Schedule I controlled substances.
Recalls & Warnings
August 05, 2016
U.S. Marshals Seize $150,00 Worth of Kratom
On August 4, 2016, the FDA announced that U.S. Marshals seized approximately $150,000 worth of dietary supplements labeled as containing kratom from Nature Therapeutics (dba Kratum Therapy). The action was taken at the request of the FDA.
Recalls & Warnings
April 16, 2016
Workout Supplement Containing Acacia Rigidula Recalled
On March 18, 2016, Nubreed Nutrition, Inc. issued a recall of all lots of its "pre-workout" supplement Undisputed which is labeled as containing Acacia rigidula, an ingredient which is not permitted in dietary supplements.
Recalls & Warnings
April 05, 2016
Muscle Enhancement Supplement Recalled
On April 5, 2016, Invisiblu International LLC issued a recall of one lot of the muscle enhancement supplement Continuum Labs LGD-Xtreme because it contains LGD-4033 Ligandrol. The risks of this ingredient are not known.
Recalls & Warnings
February 09, 2017
Herbal Supplement Found to Contain Ephedra Alkaloids Recalled
On February 7, 2017, Kingsway Trading Inc. issued a recall of Xanthium & Siler Combo (Bi Yan Pian) Dietary Supplement because it contains ephedra alkaloids.
Recalls & Warnings
November 29, 2016
Bentonite Clay Promoted for "Detoxification" Contaminated With Lead
On June 17, 2016, the FDA issued a warning letter to Best Bentonite, following a facility inspection and laboratory analysis of product sample which found the company's Best Bentonite to be contaminated with lead.
Recalls & Warnings
November 25, 2016
DMAA Product Recalled
On November 23, 2016, NutriVitaShop (a dba of Naturecom Inc.) issued a recall of its DMAA net weight 500g because it may contain DMAA.
Recalls & Warnings
March 10, 2018
Kratom Supplements Recalled Due to Salmonella Risk
On March 10, 2018, PDX Aromatics of Portland, Oregon DBA Kraken Kratom, Phytoextractum, and Soul Speciosa issued a recall of certain kratom-containing powder products because they have the potential to be contaminated with Salmonella.
News Release
January 13, 2004
ConsumerLab.com finds discrepancies in strength of CoQ10 supplements — Increasingly popular supplement, but health professionals and consumers cautioned to check brands
WHITE PLAINS, NY — January 13, 2004 — (Updated January 30) — ConsumerLab.com today announced that among the coenzyme Q10 (CoQ10) supplements it recently tested there was no detectable CoQ10 in one product.
News Release
January 09, 2004
USANA products pass ConsumerLab.com screening for substances banned from Olympics
WHITE PLAINS, NY — January 9, 2004 — ConsumerLab.com announced today that six additional products have passed its Athletic Banned Substances Screening Program. ConsumerLab.com tested the products at the request of USANA, a supplement manufacturer. ConsumerLab.
News Release
December 08, 2003
ConsumerLab.com dispels myths about HGH human growth hormone supplements sold widely on Internet
WHITE PLAINS, NY — December 8, 2003 — ConsumerLab.com has released a review of the scientific evidence behind human growth hormone (hGH or HGH) supplements.
News Release
August 28, 2003
Some problems persist with ginseng supplements but overall quality improves according to ConsumerLab.com — Test results published online today
WHITE PLAINS, NY — August 28, 2003 — In contrast to its testing three years ago in which nearly 60% of Asian and American Ginseng supplements were found to have significant problems, testing of recently purchased products by ConsumerLab.
Recalls & Warnings
March 16, 2006
Garden of Life, Maker of Primal Defense, Settles FTC Charges
On March 9, 2006, The Federal Trade Commission (FTC) announced that an operation that marketed dietary supplements sold at Whole Foods Market, GNC, the Vitamin Shoppe, and on the Internet settled FTC charges that they made deceptive advertising claims about their supplements.
Recalls & Warnings
October 18, 2005
FTC Stops False Claims about HGH Oral Spray
On October 18, 2005, the Federal Trade Commission (FTC) announced that, at its request, a federal court issued a temporary restraining order against marketers of oral sprays that supposedly contain human growth hormone (HGH) to stop them from making alleged false and deceptive claims and from ...
Recalls & Warnings
July 30, 2004
FTC Sues Florida Man for Illegal Spam and False "Human Growth Hormone" Product Claims
On July 29, 2004, The Federal Trade Commission (FTC) reported that a federal court in Chicago has issued a court order halting illegal spamming and deceptive product claims, and has also frozen the assets of Creaghan A. Harry, a resident of Boca Raton, Florida.
Recalls & Warnings
January 03, 2004
FDA to Prohibit Sales of Dietary Supplements Containing Ephedra
On December 30, 2003, the U.S. Food and Drug Administration (FDA) issued a consumer alert of its forthcoming determination that dietary supplements containing ephedra present an unreasonable risk of illness or injury, and should not be consumed.
Recalls & Warnings
February 01, 2013
DMAA Supplement Linked to Runner's Death
The cause of death of London marathon runner Claire Squires has been ruled by the investigating coroner as cardiac failure due to extreme exertion, complicated by DMAA toxicity.
Recalls & Warnings
July 27, 2012
Prescription and Street Drug Alternatives Found in Male Enhancement, Mood, and Sleep Supplements
On July 10, 2012, the FDA issued a warning letter to Evol Nutrition Associates, Inc. subsequent to a 2011 inspection which found a number of the company's products contained prescription and investigational drugs and were misbranded.
Recalls & Warnings
May 01, 2009
FDA Warns Consumers to Stop Using Hydroxycut -- Product Being Tested by ConsumerLab.com
On May 1, 2009, the U.S. Food and Drug Administration warned consumers to immediately stop using Hydroxycut products by Iovate Health Sciences Inc., of Oakville, Ontario and distributed by Iovate Health Sciences USA Inc. of Blasdell, N.Y.
Recalls & Warnings
March 01, 2013
Manufacturer of Joint, Weight Loss, Muscle Supplements and More Warned for Adulteration, Misbranding and Drug Claims
On February 4, 2013, the FDA issued a warning letter to DC Nutrition, Inc.
Recalls & Warnings
November 04, 2016
Liver Injuries Linked With Dietary Supplement Use on the Rise
The number of liver injuries associated with dietary supplement use has increased substantially in recent years, according to a recently published study in the journal Hepatology.
Recalls & Warnings
October 28, 2015
GNC Accused of Selling Supplements with Unlawful Ingredients
On October 22, 2015, Oregon Attorney General Ellen Rosenblum filed a lawsuit against supplement retailer GNC, alleging that the company sold products that were adulterated with BMPEA and picamilon, which are not lawful dietary supplement ingredients.
Recalls & Warnings
December 12, 2014
Seller of B-12, Zinc, Echinacea and More Warned for Manufacturing Violations and Drug Claims
On November 14, 2014, the FDA issued a warning letter to Scientific Botanicals Company, Inc.
News Release
May 05, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 5, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
News Release
March 06, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 6, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
News Release
October 30, 2001
Sixty percent of nutrition bars fail to meet claims in ConsumerLab.com tests — "Low Carb" bars often loaded with carbohydrates; excess sodium and saturated fat also found
WHITE PLAINS, NY — October 30, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, released results today of its Nutrition Bar Product Review.
News Release
August 15, 2001
Problems and ambiguity among alternative estrogen products reported by ConsumerLab.com — Results of soy and red clover isoflavone product testing released online today
WHITE PLAINS, NY — August 15, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Phytoestrogen Product Review. The review focused on dietary supplements made from soy and red clover isoflavones.
News Release
July 09, 2001
Many herbal sleep products lack key claimed ingredient — Results of ConsumerLab.com's testing of valerian products released on web today
WHITE PLAINS, NY — July 9, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Product Review of valerian supplements — used primarily as sleep aids and minor tranquilizers.
News Release
June 04, 2001
ConsumerLab.com reports test results of arthritis supplement — MSM; Quality found higher than for most supplements, but room for improvement remains
WHITE PLAINS, NY — June 4, 2001 — ConsumerLab.
News Release
May 07, 2001
Over forty percent of Echinacea products fail ConsumerLab.com review; Inadequate labeling and missing ingredients found common for popular herbal cold remedy
WHITE PLAINS, NY — May 7, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Product Review of echinacea supplements.
News Release
April 10, 2001
Quality of popular herbal anti-depressant found to vary; certain types of St. John's Wort supplements more likely to pass testing
White Plains, NY — April 11, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Product Review of St. John's wort supplements.
Recalls & Warnings
September 23, 2014
Maker of Joint and Weight Products Warned for Hidden Drugs, Manufacturing Violations
On September 15, 2014, the FDA issued a warning letter to West Coast Laboratories, Inc., because the company's joint health supplements, Super ArthGold and Pro ArthMax, were found to contain hidden drugs.
News Release
March 27, 2001
ConsumerLab.com expands testing of health products; success of dietary supplement evaluations leads company into broader nutrition field, beginning with popular nutrition bars
WHITE PLAINS, NY — March 27, 2001 — Having established its presence as the leading independent evaluator of the quality of dietary supplements sold in the U.S, ConsumerLab.com announced today the expansion of its work to include foods and food ingredients.
News Release
March 13, 2001
ConsumerLab.com review of vitamin E supplements finds some insufficiencies and need for clearer labeling; test results released online today
WHITE PLAINS, NY — March 13, 2001 — ConsumerLab.com today released results of its Product Review of vitamin E products. Vitamin E is one of the most popular dietary supplements in the U.S.
News Release
July 11, 2000
Pesticide contamination found in many Ginseng supplements tested by ConsumerLab.com
WHITE PLAINS, NY, July 11, 2000 — Out of 22 brands of ginseng dietary supplements evaluated by ConsumerLab.com eight were found to contain high levels of specific pesticides, some of which also contained significant levels of lead.
Recalls & Warnings
July 01, 2003
Direct Marketers of Weight Loss, Impotence, and Arthritis Supplements Charged with Deceptive Claims
On July 1, 2003, the Federal Trade Commission (FTC) announced three enforcement actions against direct marketers of weight-loss products containing ephedra. The two settlements and one complaint, filed in U.S.
Recalls & Warnings
March 04, 2003
U.S. Warns of Ephedra Risks and Proposes Warning Label for Supplements
On February 28, the Department of Health and Human Services (HHS) announced a series of actions designed to protect Americans from potentially serious risks of dietary supplement products containing ephedra.
Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.





