Product Reviews and Information for Chronic Bronchitis
Search term may appear only in full report available to members. Join now for full access.
Product Review
NAC (N-Acetyl Cysteine) Supplements Review
Choose the Best N-Acetyl Cysteine Supplement. See Our Tests of Popular NAC Supplements and Top Picks for Quality and Value.
Product Review
Echinacea Supplements Review
Many Echinacea Supplements Fail CL's Tests. Make Sure You Know What You're Getting!

Product Review
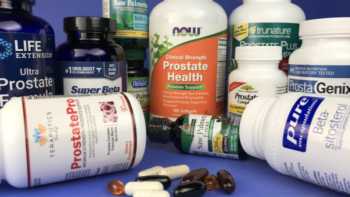
Prostate Supplements Review (Saw Palmetto and Beta-Sitosterol)
Find the Best Prostate Supplement for Symptoms of BPH. See Which Prostate Supplement Passed or Failed CL's Tests of Quality.
CL Answer
Is it true that NAC can help clear nasal passages?
Find out if N-Acetyl Cysteine (NAC) can help with post nasal drip & general sinus congestion and about supplements that may reduce cold symptoms.

CL Answer
Mullein Tea: Possible Health Effects and Safety
Mullein tea is often promoted for relieving symptoms of lung conditions, including coughing, hoarseness, sore throat, and shortness of breath. Find out if there is evidence to support these uses, and learn about possible side effects of mullein.

CL Answer
What is the difference between cysteine and cystine?
Find out the difference between cysteine and cystine amino acids, including where each is found and their functions.

CL Answer
Do any supplements help prevent or treat a cold?
Are there supplements that can help prevent or treat cold symptoms? Learn more about zinc, probiotics, vitamin C and more.

CL Answer
Are people with kidney disease at risk of getting too much potassium if they take a multivitamin? Which multivitamins are best for people with kidney disease?
Learn if people with kidney disease are at increased risk of getting too much potassium from multivitamins, find out which multivitamins do not contain potassium, and learn what other products should be avoided by people restricting potassium intake.

CL Answer
Will pregnenolone help slow down aging or reduce chronic pain?
Learn more about the steroid hormone pregnenolone, including its effects in the body and on aging and chronic pain.

CL Answer
Do any supplements help with pancreatitis? Do any cause pancreatitis?
Find out if supplements including digestive enzymes, antioxidants, or medium-chain triglycerides are beneficial for chronic pancreatitis, and learn which supplements might worsen symptoms.
CL Answer
Do any supplements help for varicose veins or chronic venous insufficiency?
Find out which supplements help for varicose veins and chronic venous insufficiency, including citrus bioflavonoids and grape seed extract.

News Release
September 26, 2024
What’s Really In Shilajit Supplements? ConsumerLab Tests Reveal Amounts of Fulvic Acid & Heavy Metals
White Plains, NY, September 26, 2024 -- Shilajit is a tar-like substance created by decomposed plant material and found in mountain rocks.
CL Answer
Can vitamins or other supplements cause a change in the ability to taste, or even a loss of taste?
Find out if supplements or vitamin deficiencies can cause changes in taste, loss of taste or smell. Information about vitamin D, zinc, vitamins A, B6, B12 and others. Plus medications and medical conditions that can alter taste and smell. ConsumerLab.com's answer explains.

News Release
March 11, 2019
Best NAC (N-acetyl cysteine) Supplements Identified by ConsumerLab
White Plains, New York, March 11, 2019 — NAC (N-acetyl cysteine) supplements are promoted for many uses, including "liver support," "immune support," and reducing symptoms of the flu and flare-ups of chronic bronchitis.
CL Answer
Which supplements and foods help relieve constipation and which can cause constipation?
Find out which supplements can cause constipation, including iron, calcium, protein powders and drinks and others. ConsumerLab.com's answer explains.

CL Answer
Are antioxidant supplements good or bad for you?
Learn more about whether antioxidants like astaxanthin, beta-carotene, coenzyme Q10, curcumin, resveratrol, selenium, vitamin A, vitamin C and vitamin E are safe and if they help prevent chronic diseases.

Recalls & Warnings
December 08, 2017
Limbrel Capsules for Osteoarthritis Linked With Potentially Life-Threatening Health Risk, FDA Warns
On December 4, 2017, the FDA urged consumers not to take Limbrel (Primus Pharmaceuticals), a capsule marketed to manage the metabolic processes associated with osteoarthritis, because it has been associated with serious adverse events, including drug-induced liver injury and a lung ...
News Release
December 09, 2014
Round-up of Recent Product Tests by ConsumerLab.com -- Results for B Vitamin Supplements & Energy Drinks, Garlic, NAC (N-acetyl cysteine), Iron and Omega-7 Fatty Acids
White Plains, New York, December 9, 2014 — In recent months, ConsumerLab.com has published test results for a variety of popular supplements, including B vitamins supplements and energy drinks, NAC (N-acetyl cysteine), iron and garlic. In addition, ConsumerLab.
Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.
Recalls & Warnings
December 14, 2023
Pain Relief Tea Recalled, Found to Contain Prescription Drugs
On December 13, 2023, WS Global issued a recall of all lots of the company’s Himalayan Pain Relief Tea after products were found to contain diclofenac and dexamethasone, prescription drugs that are not listed on the product’s label.
Recalls & Warnings
April 02, 2021
FDA Warns Sellers of Prostate, Reishi, Immune Products, and More
On March 16, 2021, the FDA issued warning letters to two companies following reviews of their websites which found statements made about the companies' products to be drug claims.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
November 07, 2015
Seller of "NaturalDoctor" Vitamin C, Echinacea and More Warned for Manufacturing Violations
On October 16, 2015, the FDA issued a warning letter Sound Healing Arts, PC, dba Grounds for Tea, LLC, following a facility inspection which found the company's products, including NaturalDoctor Vitamins C & K3, NaturalDoctor Goldenseal & Echinacea Plus, NaturalDoctor Centella ...
Recalls & Warnings
May 03, 2013
Maker of Omega-3, Saw Palmetto, St. John’s Wort Supplements And More Warned For Manufacturing Violations and Drug Claims
On March 19, 2013, the FDA issued a warning letter to Sunset Natural Products Inc.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
May 30, 2020
FTC Sends Refund Checks for TrueAloe and AloeCran
On May 26, 2020, the FTC announced it is mailing 22,581 checks totaling more than $470,000 to consumers who purchased deceptively marketed supplements, TrueAloe and AloeCran.
Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
Recalls & Warnings
March 19, 2019
FDA Calls Promotion of Nyloxin Homeopathic Products for Pain a "Health Fraud Scam"
On March 11, 2019, the FDA issued a warning letter to Nutra Pharma Corp. following a review of the company's website (www.Nyloxin.com) and social media sites found that the company was making drug claims about its homeopathic products promoted for arthritis and cancer pain.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
September 14, 2016
Seller of Aloe, Prostate and Joint Supplements Warned for Manufacturing Violations
On April 8, 2016, the FDA issued a warning letter to Salud Natural Entrepreneurs, Inc.
Recalls & Warnings
May 03, 2013
Homeopathic Company Warned For Drug Claims and Improper Labeling
On March 12, 2013, the FDA issued a warning letter to Natural Medicine Associates, Inc.
Recalls & Warnings
July 11, 2014
Maker of Cough and Sleep "Remedies" Warned for Drug Claims
On June 27, 2014, the FDA issued a warning letter to Zarbee's, Inc.
Recalls & Warnings
May 06, 2014
Seller of Flaxseed, Ginkgo and More Warned for Manufacturing Violations and Drug Claims
On April 18, 2014, the FDA issued a warning letter to Iowa Select Herbs, LLC, following a facility inspection which found the company's products, including Flax Seed, Holy Basil, Papaya Leaf Extract, and Ginkgo Leaf Extract products, to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
December 22, 2005
Health Canada Warns Consumers Not to Take Chaparral
On December 21, 2005, Health Canada (Canada's health ministry) warned consumers not to ingest the herb chaparral in the form of loose leaves, teas, capsules or bulk herbal products because of the risk of liver and kidney problems.
Recalls & Warnings
August 30, 2012
Supplement Company Warned For Medical Claims and Misbranding Of Omega-3, CoQ10, Noni Juice And More
On July 12, 2012, the FDA issued a warning letter to Alfa Vitamins Laboratories, Inc.
Recalls & Warnings
November 28, 2014
Seller of Herbal Extracts Warned for Manufacturing Violations, Drug Claims
On November 3, 2014, the FDA issued a warning letter to Avena Botanicals, Inc.
Recalls & Warnings
September 10, 2014
Seller of Joint Pain Supplement Warned for Drug Claims
On August 24, 2014, the FDA issued a warning letter to CreAgri, Inc., following a website review, which found statements made about the company's products, including Olivenol plus Easeflex, Olivenol plus Essence Capsules, and Olivenol plus Essence Elixer, to be drug claims.
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
September 13, 2013
Metagenics Warned For Misbranding of Medical Foods and Drug Claims
On August 13, 2013, the FDA issued a warning letter to Metagenics, Inc.
Recalls & Warnings
March 16, 2006
Garden of Life, Maker of Primal Defense, Settles FTC Charges
On March 9, 2006, The Federal Trade Commission (FTC) announced that an operation that marketed dietary supplements sold at Whole Foods Market, GNC, the Vitamin Shoppe, and on the Internet settled FTC charges that they made deceptive advertising claims about their supplements.
Recalls & Warnings
July 15, 2014
Vita Springs Health Warned for Manufacturing Violations and Drug Claims
On June 24, 2014, the FDA issued a warning letter to Albert Max Inc.





