Product Reviews and Information for Chronic Obstructive Pulmonary Disease (COPD)
Search term may appear only in full report available to members. Join now for full access.
CL Answer
Which is the best pulse oximeter for home use?
Best pulse oximeter for home use (COVID-19, asthma and COPD). Differences between home use and FDA-approved medical pulse oximeters.

Product Review
Quercetin & Rutin Supplements Review
Quality's a Concern With Quercetin and Rutin Supplements -- Only 17% of Claimed Amount In One

Product Review
NAC (N-Acetyl Cysteine) Supplements Review
Choose the Best N-Acetyl Cysteine Supplement. See Our Tests of Popular NAC Supplements and Top Picks for Quality and Value.
Product Review
Muscle & Workout Supplements Review (Creatine and Branched-chain Amino Acids)
Do Creatine and BCAAs Really Improve Strength and Recovery?

CL Answer
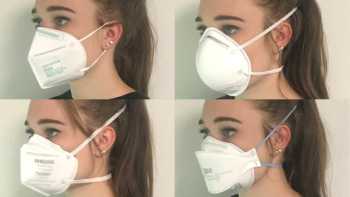
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
CL Answer
Are people with kidney disease at risk of getting too much potassium if they take a multivitamin? Which multivitamins are best for people with kidney disease?
Learn if people with kidney disease are at increased risk of getting too much potassium from multivitamins, find out which multivitamins do not contain potassium, and learn what other products should be avoided by people restricting potassium intake.

CL Answer
Can vitamins or other supplements cause a change in the ability to taste, or even a loss of taste?
Find out if supplements or vitamin deficiencies can cause changes in taste, loss of taste or smell. Information about vitamin D, zinc, vitamins A, B6, B12 and others. Plus medications and medical conditions that can alter taste and smell. ConsumerLab.com's answer explains.

Recalls & Warnings
March 23, 2023
FDA Warns Herbal Vitality for Promoting Supplements to Treat Cold & Flu, Kidney Stones & More
On March 7, 2023, the FDA issued a warning letter to Herbal Vitality, Inc.
Recalls & Warnings
May 08, 2021
Seller of Immune Bio Green Cell Warned by FDA
On March 30, 2021, the FDA issued a warning letter to Immune & Genetics Protocols, LLC following a review of the company's websites, which found statements made about the company's product Immune Bio Green Cell to be drug claims.
Recalls & Warnings
April 18, 2022
Moringa Concern: Blood Clots
A 63-year-old woman in New York with type 2 diabetes developed a blood clot in the lungs (i.e. pulmonary embolism) that her physicians suspect may have been caused by the use of a supplement containing Moringa oleifera extract (Ebhohon, Int J Emerg Med 2022).
Recalls & Warnings
June 20, 2003
FTC Charges Marketers of Seasilver with False and Deceptive Claims
On June 19, 2003, The Federal Trade Commission (FTC) and the Food and Drug Administration (FDA) announced coordinated actions against two companies - both charged with promoting the dietary supplement "Seasilver" with unsubstantiated medical claims. The agencies' actions against Seasilver USA, Inc.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
August 30, 2012
Supplement Company Warned For Medical Claims and Misbranding Of Omega-3, CoQ10, Noni Juice And More
On July 12, 2012, the FDA issued a warning letter to Alfa Vitamins Laboratories, Inc.
Recalls & Warnings
April 11, 2013
Glutathione Supplement Maker Warned For Manufacturing Violations and Drug Claims
On April 5, 2013, the FDA issued a warning letter to The Glutathione Corporation following a facility inspection which found the company's supplements, including Ultrathione 500 Glutathione, Ultrathione 1000 Sports Pack, Ultrathione Performance, Ultrathione Extreme Performance, Ultrathione Health ...
Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
Recalls & Warnings
July 29, 2009
FDA Warns Against Body Building Supplements with Steroid-like Compounds
On July 28, 2009, the U.S. FDA notified the public about new safety information concerning products marketed for body building and increasing muscle mass.
Recalls & Warnings
September 13, 2013
Metagenics Warned For Misbranding of Medical Foods and Drug Claims
On August 13, 2013, the FDA issued a warning letter to Metagenics, Inc.
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
August 08, 2017
Study Shows Severe Risk in Drinking Hydrogen Peroxide
An analysis of records from the National Poison Data System from a 10-year period ending in 2011 identified 294 reported incidents of people developing adverse symptoms after ingesting 35% (high-concentration) hydrogen peroxide (H202). Among these, 41 (13.
Recalls & Warnings
July 12, 2017
More Bodybuilding Supplements Recalled
On July 11, 2017, Andropharm recalled two bodybuilding supplements, Sten Z and M1 Alpha, because these products contain derivatives of anabolic steroids.
Recalls & Warnings
June 20, 2017
Beware of Bodybuilding Supplements, FDA Warns
On June 20, 2017, the FDA warned consumers to beware of bodybuilding products because a number of these products have been found to contain steroids or steroid-like substances. Such products could cause serious and even life-threatening health risks, including:
Recalls & Warnings
March 16, 2006
Garden of Life, Maker of Primal Defense, Settles FTC Charges
On March 9, 2006, The Federal Trade Commission (FTC) announced that an operation that marketed dietary supplements sold at Whole Foods Market, GNC, the Vitamin Shoppe, and on the Internet settled FTC charges that they made deceptive advertising claims about their supplements.
Recalls & Warnings
January 16, 2016
Seller of Liver, Lung Support Supplements Warned for Drug Claims
On January 4, 2016, the FDA issued a warning letter to Tibetan Herbal Balance, Inc.





