Product Reviews and Information for Chronic Pain
Search term may appear only in full report available to members. Join now for full access.
CL Answer
Will pregnenolone help slow down aging or reduce chronic pain?
Learn more about the steroid hormone pregnenolone, including its effects in the body and on aging and chronic pain.

Product Review
CBD Oils, Softgels, Gummies, Creams & Salves Review
See How Much CBD and THC We Found in Products.

CL Answer
I have IBS and don't want to take medication if I can help it. I've heard that peppermint works for IBS, but is there good research to support this?
Information about using peppermint oil for irritable bowel syndrome (IBS), including clinical evidence, dosage, safety and more.

CL Answer
Do any supplements help treat or reduce the pain of plantar fasciitis (heel pain)?
Plantar fasciitis causing heel pain has, in rare cases, been reported as a symptom of vitamin D deficiency. Other supplements that have been proposed to help include vitamin C, zinc, curcumin (from turmeric), bromelain, collagen and glucosamine. Find out what the evidence shows, and what other treatments may help.

CL Answer
Can CBD cause a drop in blood pressure?
Find out if CBD (cannabidiol) can lower blood pressure or affect heart rate, plus information about other CBD side effects, drug interactions and safety concerns. ConsumerLab.com's answer explains.
CL Answer
Is kratom too dangerous to use?
Find out if kratom is safe/legal to use, including potential adverse effects, such as agitation, rapid heartbeat, nausea, drowsiness, seizures, and possible drug interactions.

CL Answer
What are the health benefits of palmitoylethanolamide (PEA), and is it safe?
Palmitoylethanolamide (PEA) is commonly promoted for its anti-inflammatory and pain-relieving effects. Find out if it works.

Recalls & Warnings
December 14, 2023
Pain Relief Tea Recalled, Found to Contain Prescription Drugs
On December 13, 2023, WS Global issued a recall of all lots of the company’s Himalayan Pain Relief Tea after products were found to contain diclofenac and dexamethasone, prescription drugs that are not listed on the product’s label.
Product Review
St. John's Wort Supplements Review
Find the Best St. John's Wort Supplement. Only 40% of St. John's Wort Supplements Pass Tests & Strength Varies Widely.

CL Answer
Do any supplements help with pancreatitis? Do any cause pancreatitis?
Find out if supplements including digestive enzymes, antioxidants, or medium-chain triglycerides are beneficial for chronic pancreatitis, and learn which supplements might worsen symptoms.
CL Answer
Does red and near infrared light therapy reduce pain, improve skin or cognition, or have other benefits? Is it safe?
Red light and near infrared light therapy devices are promoted for numerous conditions, including acne, fibromyalgia, pain, cognitive function and other uses. Find out if these devices work and if they are safe.

CL Answer
I was told I have a MTHFR gene mutation. Do I need to take methylated forms of B vitamins, such as methylfolate and methylcobalamin?
MTHFR gene mutation can impact B vitamin bioavailability. Learn more about this and which deficiencies are more likely to occur.

CL Answer
Do any supplements help for varicose veins or chronic venous insufficiency?
Find out which supplements help for varicose veins and chronic venous insufficiency, including citrus bioflavonoids and grape seed extract.

Product Review
Acetyl-L-Carnitine Supplements Review
Choose the Best Acetyl-L-Carnitine Supplement. Don't Overpay! CL Identifies Quality Acetyl-L-Carnitine Supplements at the Best Value.
.jpg?size=small)
Product Review
NAC (N-Acetyl Cysteine) Supplements Review
Choose the Best N-Acetyl Cysteine Supplement. See Our Tests of Popular NAC Supplements and Top Picks for Quality and Value.
Product Review
Turmeric and Curcumin Supplements and Spices Review
See Our Top Picks Among Turmeric Products

CL Answer
Adrenal Fatigue and Adrenal Support Supplements: Do they work and are they safe?
Find out what's really in supplements for adrenal support and adrenal fatigue, including herbal adrenal support supplements and bovine derived adrenal support supplements. Find out if they really work and if they are safe. ConsumerLab.com's answer explains.

CL Answer
My dog is getting older and his veterinarian recently recommended giving him a glucosamine supplement for his joints. Has ConsumerLab.com tested these, or other supplements for pets?
Find out our test results on glucosamine for dogs and cats as well as other supplements for pets.

Product Review
Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, Boswellia, Collagen and Turmeric)
18% of Joint Health Supplements Failed to Pass Our Tests. See Which Passed or Failed, and Our Top Picks.

Product Review
Melatonin Supplements Review
Trouble Sleeping? See CL's Tests of Melatonin Supplements and Top Picks.
CL Answer
After taking a curcumin supplement, I started getting chronic diarrhea which went away when I stopped taking it. Can curcumin supplements cause diarrhea?
Learn more about the side effects of curcumin supplements, including diarrhea and other gastrointestinal discomfort.

CL Answer
Are people with kidney disease at risk of getting too much potassium if they take a multivitamin? Which multivitamins are best for people with kidney disease?
Learn if people with kidney disease are at increased risk of getting too much potassium from multivitamins, find out which multivitamins do not contain potassium, and learn what other products should be avoided by people restricting potassium intake.

Product Review
Alpha-Lipoic Acid Supplements Review
Choose the Best Alpha-Lipoic Acid Supplement — See the Amounts of Active "R-Isomer" We Found

CL Answer
Is topical menthol, such as Biofreeze, safe and effective for pain relief?
Menthol is commonly used in topical creams and gels for pain relief. Find out if it works and if it's safe.
CL Answer
10 Supplements That May Help Reduce Nighttime Urination (And 5 That May Not)
Waking up more than once during the night to use the bathroom? Learn more about the 9 supplements that may help and 5 others that don't.

CL Answer
Which supplements help to improve energy and decrease fatigue?
Energy supplements and vitamins - Some can help increase energy and reduce fatigue, including CoQ10, curcumin/turmeric, cocoa, and B vitamins.

CL Answer
Which supplements and foods help relieve constipation and which can cause constipation?
Find out which supplements can cause constipation, including iron, calcium, protein powders and drinks and others. ConsumerLab.com's answer explains.

CL Answer
Can vitamins or other supplements cause a change in the ability to taste, or even a loss of taste?
Find out if supplements or vitamin deficiencies can cause changes in taste, loss of taste or smell. Information about vitamin D, zinc, vitamins A, B6, B12 and others. Plus medications and medical conditions that can alter taste and smell. ConsumerLab.com's answer explains.

CL Answer
Is Relief Factor likely to relieve aches and pains, and is it safe?
Find out if the dietary supplement Relief Factor is likely to help relieve aches and pains, if it is safe, and if it is worth the cost.

CL Answer
Risks of Too Many Vitamins & Supplements
Taking too many vitamin supplements -- or taking too much of a particular supplement -- can have short and long-term adverse effects.

Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
CL Answer
7 Tips to Avoid Getting a Pill Stuck in Your Throat
Some pills can be difficult to swallow and can even become lodged in the throat or cause damage to the esophagus. Learn seven practical tips to help avoid getting pills stuck in the throat.

CL Answer
What is Pycnogenol, does it work, and is it the same as other pine bark extracts?
Learn more about Pycnogenol, including evidence from clinical studies on blood clots, osteoarthritis, cognition, vision, tinnitus, and the skin and safety.

CL Answer
Can any supplements be used to reduce inflammation in people sensitive to NSAIDs such as ibuprofen, high-dose aspirin, or naproxen?
Find out if supplements such as turmeric, fish oil, MSM (methylsulfonylmethane), SAMe (S-adenosyl-methionine), boswellia, ginger, white willow bark or arnica may, or may not, be helpful to people sensitive to NSAIDs.

CL Answer
Can baking soda really help treat rheumatoid arthritis?
Find out if there is evidence showing that baking soda (sodium bicarbonate) can help for rheumatoid arthritis. Plus, get information about safety and drug interactions with baking soda. ConsumerLab.com's answer explains.

CL Answer
ConsumerLab’s Review of ION* Gut Support
ConsumerLab's Review of ION Gut Support (formerly Restore), the liquid humic extract supplement derived from soil. Learn about the evidence for ION Gut Support for on digestion and gut health, reducing inflammation, and other uses, dosage, cost, and safety.

CL Answer
Can drinking coffee weaken bones or make arthritis worse?
Learn about the effects of coffee intake on bone mineral density, risk of osteoporosis, and risk of fracture. Also, find out if drinking coffee might increase the risk of arthritis or worsen symptoms.

CL Answer
Conolidine: Evidence for Pain Relief, Safety, and CONOCB2™ Products Explained
Find out if conolidine, the main ingredient in CONOCB2 Blend, offers natural pain relief without the side effects of opioids. Also learn about the top conolidine products available today.

CL Answer
Do any supplements, like Nervive, help with nerve pain, like sciatica, diabetic neuropathy or postherpetic neuralgia?
Find out which supplements for nerve pain, including fish oil and alpha-lipoic acid, are effective for sciatica, diabetic neuropathy, or postherpetic neuralgia.

CL Answer
Can vitamin C reduce the pain of shingles?
Can taking vitamin C for shingles help reduce pain and post-shingles pain, otherwise known as post-herpetic neuralgia? ConsumerLab.com's answer explains.

CL Answer
Is sublingual vitamin B-12, which is placed under the tongue and allowed to dissolve, really better than the pill form?
Learn the difference between sublingual vitamin B-12, vitamin B-12 pills and more through information including clinical evidence.

CL Answer
Which supplements help with arthritis?
Learn more about supplements for joint health, including products such as Wobenzym and Zyflamend and ingredients such as collagen, curcumin, glucosamine and chondroitin, hyaluronic acid, magnesium, omega-3 fatty acids, palmitoylethanolamide (PEA), SAMe, and others.

CL Answer
Do any supplements help reduce menstrual pain or symptoms of premenstrual syndrome (PMS)?
Find out which supplements help reduce premenstrual syndrome (PMS) symptoms, such as mood swings, pain, bloating, irritability and food cravings.

CL Answer
Does chuchuhuasi reduce inflammation or back pain?
Find out if chuchuhuasi, an herb from the Amazon rainforest, can decrease inflammation or reduce back pain. ConsumerLab.com's answer explains.

CL Answer
Can any supplements improve or worsen symptoms of fibromyalgia?
Find out if any supplements may help improve fibromyalgia symptoms, learn which supplements do not seem beneficial, and find out if any supplements worsen symptoms.

Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
Recalls & Warnings
March 19, 2019
FDA Calls Promotion of Nyloxin Homeopathic Products for Pain a "Health Fraud Scam"
On March 11, 2019, the FDA issued a warning letter to Nutra Pharma Corp. following a review of the company's website (www.Nyloxin.com) and social media sites found that the company was making drug claims about its homeopathic products promoted for arthritis and cancer pain.
CL Answer
Is it true that low-carb or Mediterranean diets help for gastroesophageal reflux disease (GERD)?
Find out if a low-carb diet, such as the Mediterranean diet, or lifestyle changes help with gastroesophageal reflux disease (GERD).

CL Answer
What are the health benefits of serrapeptase?
Serrapeptase is a proteolytic enzyme. Learn more about its health effects, including whether evidence supports its use for pain, inflammation, and heart health.

CL Answer
Is UpWellness Golden Revive + for Joint Support Worth It?
UpWellness Golden Revive + claims to offer relief for inflammation, muscle tension, and nerve pain. Find out if research supports these claims and if it's worth its price.

CL Answer
Are antioxidant supplements good or bad for you?
Learn more about whether antioxidants like astaxanthin, beta-carotene, coenzyme Q10, curcumin, resveratrol, selenium, vitamin A, vitamin C and vitamin E are safe and if they help prevent chronic diseases.

CL Answer
Do Vital 3 collagen drops reduce joint pain?
Learn more about Vital 3 collagen drops withundenatured type II collagen, and if they can help joints move better and feel better.
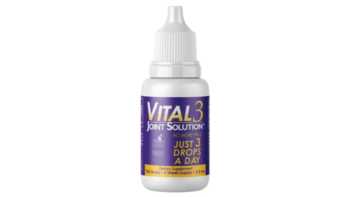
CL Answer
Do any supplements help for irritable bowel syndrome (IBS)?
IBS supplements may help reduce irritable bowel symptoms such as pain, diarrhea and constipation. Supplements include probiotics, melatonin, and flaxseed.

CL Answer
Does cat’s claw have any health benefits and is it safe?
Cat's claw is promoted for numerous conditions, including arthritis (including osteoarthritis and rheumatoid arthritis), Alzheimer's disease, viral infections, boosting immune response to vaccination, and reducing side effects of chemotherapy. Find out if it works and if it's safe.

CL Answer
Does Arthrozene help with joint pain, and is it safe?
Arthrozene by Fisico is promoted for joint stiffness, soreness and discomfort. Find out if it is likely to help and if it is worth the cost.

CL Answer
Does collagen taken as a supplement help with arthritis? I'm seeing it in products for joint health.
Learn more about collagen supplements, including evidence from clinical studies on arthritis and muscle strength and safety.

CL Answer
What is UC-II and does it help joints?
What is UC-II (undenatured type 2 collagen - InterHealth Nutraceuticals) and do clinical studies show that it works for joint health?

CL Answer
OmegaXL Review: Does It Really Work for Joint and Muscle Pain?
Read our in-depth review of OmegaXL to see if it really helps with joint and muscle pain.
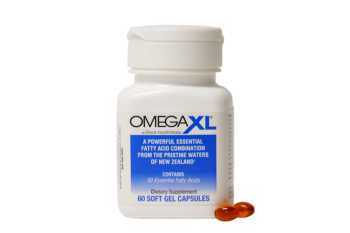
CL Answer
Is hyaluronic acid helpful for osteoarthritis?
Hyaluronic acid supplement information including joint pain, osteoarthritis, clinical evidence, dosage and more.

CL Answer
What are the health benefits of tart cherry juice?
See the evidence for tart cherry juice health benefits from clinical studies. Find out if tart cherry has anti-inflammatory effects, if it can improve sleep, lower high blood pressure, or help for muscle pain and osteoarthritis.

CL Answer
Do arnica pills, gels, creams, or sprays reduce bruising, pain, or swelling?
Find out if arnica pills, gels, creams, or sprays can reduce bruising, pain, or swelling, help with varicose veins, or muscle pain after exercise.

CL Answer
Are intravenous (IV) vitamin infusions safe and effective?
Intravenous (IV) vitamin infusions are touted by celebrities and promoted by wellness clinics for numerous conditions. Find out if they work and if they are safe.

CL Answer
Which supplements can cause diarrhea?
Can supplements like magnesium cause diarrhea? Find out which supplements may cause diarrhea, such as vitamin B12, curcumin, magnesium, vitamin C and fish oil.

CL Answer
What is lumbrokinase, does it have heart benefits, is it safe, and what should you look for, or avoid, in a lumbrokinase supplement?
Lumbrokinase is marketed as promoting healthy circulation and supporting heart health. Find out how and if lumbrokinase supplements actually work, possible safety concerns, and what to consider when choosing a lumbrokinase supplement.

CL Answer
Schisandra Berry Supplements: Health Benefits and Safety
Schisandra berry extracts and supplements are promoted to improve mood and sleep, liver health, muscle strength, and symptoms of menopause. Find out if they work and are safe, including side effects and drug interactions.

CL Answer
What are the side effects of glucosamine and chondroitin?
Learn the side effects of glucosamine and chondroitin supplements for joint health and joint pain, including stomach upset, headache, and many others. ConsumerLab.com's answer explains.

CL Answer
Do diamine oxidase (DAO) supplements for histamine intolerance really work?
DAO supplement info for histamine intolerance, including diamine oxidase safety, evidence from clinical studies, and dosage.

CL Answer
What is Prelief? Does it really help for heartburn and/or bladder pain from acidic foods?
Prelief information, including what it has been claimed to do (such as helping with painful bladder, frequent urination, heartburn or indigestion from acidic foods), clinical evidence, and safety.
CL Answer
How can I avoid aconite – a toxin – in Aconitum supplements?
Aconite is a highly toxic alkaloid found in Aconitum plants ( also called monkshood, wolfsbane, or devil's hood). Aconite poisoning can cause low blood pressure, chest pain, increased or decreased heart rate, and irregular heart rhythm that can lead to cardiac arrest and death. Find out which supplements may contain aconite and how to avoid it.

CL Answer
Do any supplements, foods, or oils help reduce muscle pain, muscle cramps, or nighttime leg cramps?
Find out which supplements, and topical products such as magnesium creams, can help reduce muscle pain, leg cramps, and nighttime leg cramps, and which may worsen muscle cramps. Also, learn about vitamin and mineral deficiencies and common medications that can cause muscle cramps and nocturnal leg cramps.
CL Answer
Does ergothioneine help prevent age-related problems such as impaired cognition, Alzheimer’s disease, Parkinson’s disease or cataract, and is it safe?
Learn about ergothioneine and its potential use for cognitive impairment, dementia, Alzheimer's disease, Parkinson's disease, cataract, or kidney disease, as well as its safety and cost of supplements containing this ingredient.

CL Answer
What are the health benefits of D-ribose and is it safe?
Find out if D-ribose supplementation is beneficial for heart failure, exercise performance, and fibromyalgia and learn if it is safe.

CL Answer
Akkermansia muciniphila: Health Benefits and Safety
Akkermansia muciniphila has gained attention for its potential benefits in managing insulin resistance, diabetes, weight loss, cancer, and other health conditions, but does it really work, and is it safe? Find out.

Recalls & Warnings
May 30, 2020
FTC Sends Refund Checks for TrueAloe and AloeCran
On May 26, 2020, the FTC announced it is mailing 22,581 checks totaling more than $470,000 to consumers who purchased deceptively marketed supplements, TrueAloe and AloeCran.
Recalls & Warnings
December 11, 2020
FDA Warns Seller of Curcumin and Cholesterol Supplements
On November 20, 2020, the FDA issued a warning letter to Natures Boost LLC following a review of the company's websites, which found statements made about the company's products Blood Boost Formula and Turmeric Curcumin to be drug claims.
Recalls & Warnings
May 14, 2021
Maker of Care by Design CBD Settles Charges of Unsupported Claims
On May 5, 2021, Cannacraft, Inc.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Joint and Hair Loss Products
On December 22, 2020, the FDA issued a warning letter to Speedwinds Nutrition, Inc. following a review of the company's website, which found statements made about the company's products Synotrex and Sephren to be drug claims.
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
October 22, 2019
Seller of TrueAloe and AloeCran Settles Charges of Making False Claims
NatureCity, LLC, has agreed to settle Federal Trade Commission (FTC) charges that they deceived consumers by making false claims about their TrueAloe and AloeCran products.
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
November 06, 2023
FDA Warns Consumers Not to Use Dr. Ergin’s SugarMD Advanced Glucose Support, Found to Contain Prescription Drugs
On November 3, 2023, the FDA warned consumers not to purchase or use Dr. Ergin’s SugarMD Advanced Glucose Support products after FDA laboratory analysis found the supplement to contain glyburide and metformin.
Recalls & Warnings
December 26, 2024
Blood Sugar Supplement Recalled Due to Undeclared Metformin and Glyburide
On December 16, 2024, Shoppers- Plaza recalled all lots of Fouzee Sugarlin Herbal Formula capsules because they were found to contain undeclared metformin and glyburide.
Recalls & Warnings
January 28, 2025
Shatavari Powder Recalled Due to Risk of Lead Contamination
On January 27, 2025, New York Wholesale Group recalled Zaarah Herbals shatavari powder because of potential lead contamination.
Recalls & Warnings
September 15, 2025
Lead Found in Organic Ground Cinnamon
On September 12, 2025, the FDA warned consumers not to buy or use Jiva Organics Organic Cinnamon Powder because it contains elevated levels of lead. The FDA warned consumers that exposure to this product could be unsafe. Testing by the agency found Jiva Organics to contain 2.
Recalls & Warnings
February 22, 2024
Pacific BioLogic Co. Warned by FDA for Manufacturing Violations
On December 21, 2023, the FDA issued a Warning Letter to Curtis Jacquot, dba Pacific BioLogic Co.
Recalls & Warnings
August 14, 2023
Liver Injury Linked to "Immune Booster" Supplement Sold on Amazon
A 27-year-old woman in Pennsylvania was admitted to intensive care for drug-induced liver injury after taking Prodigy Life HRP-AID, a supplement sold on Amazon and promoted as an "immune system booster" to reduce the intensity and frequency of cold sore outbreaks, according to a ...
News Release
July 26, 2023
Best Collagen Supplements for Skin and Joints, According to ConsumerLab Tests
White Plains, New York, July 26, 2023 — Collagen supplementation may modestly reduce wrinkles and the appearance of cellulite, and moderately decrease joint and tendon pain and improve flexibility in people with osteoarthritis.
Recalls & Warnings
November 05, 2021
Herb Recalled Due to Risk of Lead, Cadmium Contamination
On November 2, 2021, Murray Int'l Trading issued a recall of Herbal Doctor brand Angelicae Sinensis due to potential risk it may contain elevated levels of lead and cadmium.
Recalls & Warnings
November 16, 2023
Toxic Levels of Lead Found in Cinnamon Applesauce
WanaBana, Schnucks, and Weis apple cinnamon fruit puree pouches have been recalled after being linked, as of December 5, 2023, to 64 reports of illness, including four children with potential lead toxicity.
Recalls & Warnings
July 31, 2018
FDA Warns Seller of Joint Health and Cholesterol Supplements
On July 13, 2018, the FDA issued a warning letter to GC Natural, following a facility inspection which found a number of the company's products, including Red Pyrola Plus + Advanced Joint Formula Capsules, CSDP GOLD Capsules, Rejeune Optimal Kidney & Liver Support Extract balls, C.
Recalls & Warnings
August 02, 2016
Seller of Whey Protein Warned for Manufacturing Violations, Drug Claims
On July 22, 2016, the FDA issued a warning letter to New Horizon Nutraceuticals, LLC, following a facility inspection which found the company's product, One World Whey Protein Power Food to be adulterated because it was prepared, packed, or held under conditions that violate Current ...
Recalls & Warnings
December 19, 2024
Force Forever Joint Pain Supplement Recalled
On December 12, 2024, GNMart INC recalled all lots of Force Forever, a supplement promoted for joint pain, because FDA tests found it to contain undeclared diclofenac and dexamethasone.
Recalls & Warnings
October 08, 2024
AK Forte Joint Pain Supplement Recalled
On October 8, 2024, C&A Naturistics voluntarily recalled all lots of AK Forte, 400 mg tablets because FDA laboratory analysis found products to contain undeclared diclofenac, dexamethasone, and methocarbamol.
Recalls & Warnings
December 22, 2021
FDA Warns Seller of Liquid Magnesium, B-12, Berberine & More
On December 9, 2021, the FDA issued a warning letter to Wholly Liquid Nutritional Supplements LLC because it found statements on the company's website and social media about its products, including SpiroLaze, BioLaze, LiquiLaurin, VIT-B12, DeStress, and Omega Plus, to be ...
News Release
March 04, 2020
CBD Most Often Used for Pain, Then Sleep, According to CL Survey. Half of Users Think It Works.
White Plains, New York, March 4, 2020 — A recent survey of 9,782 people who use dietary supplements found that 14.5% of respondents purchased CBD (or hemp extract, which is a source of CBD) in 2019. The survey asked those people what they used CBD for and whether or not they thought it worked.
News Release
October 04, 2019
ConsumerLab Tests Reveal Best Collagen Supplements for Wrinkles and Joints
White Plains, New York, October 4, 2019 — Collagen supplements are promoted to reduce wrinkles and decrease joint pain and stiffness, but do they really work, and if so, which products on the market provide the best quality collagen and the best value? To find out, ConsumerLab carefully ...
Recalls & Warnings
May 03, 2013
Maker of Omega-3, Saw Palmetto, St. John’s Wort Supplements And More Warned For Manufacturing Violations and Drug Claims
On March 19, 2013, the FDA issued a warning letter to Sunset Natural Products Inc.
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
October 26, 2023
Joint Pain Supplements Recalled Due to Undeclared Drug Ingredients
On October 22, 2023, Botanical-Be recalled of all lots of the company’s Kuka Flex Forte, Artri King, and Reumo Flex capsule products after FDA laboratory analysis found them to contain undeclared diclofenac.
Recalls & Warnings
September 25, 2025
FDA Warns Consumers Not to Use Joint Pain Supplement FTX PLUS
On September 5, 2025, the FDA advised consumers not to purchase or use FTX PLUS, a product promoted for joint pain on various websites, including Walmart and possibly in some retail stores.
Recalls & Warnings
September 05, 2024
Don’t Buy or Use Umary and Amazy Supplements, Warns FDA
On September 5, 2024, the FDA warned consumers not to purchase or use Umary Hyaluronic Acid or Amazy Hyaluronic Acid supplements because testing by the agency found them to contain diclofenac and omeprazole.
News Release
March 11, 2019
Best NAC (N-acetyl cysteine) Supplements Identified by ConsumerLab
White Plains, New York, March 11, 2019 — NAC (N-acetyl cysteine) supplements are promoted for many uses, including "liver support," "immune support," and reducing symptoms of the flu and flare-ups of chronic bronchitis.
Recalls & Warnings
November 21, 2024
Umary Hyaluronic Acid Sold on Amazon Recalled
On November 20, 2024, MXBBB issued a recall of one lot of Umary Hyaluronic Acid or Amazy Hyaluronic Acid supplements, which were sold on Amazon.
News Release
June 21, 2017
Which Magnesium Is Best? ConsumerLab.com Tests Popular Brands and Reveals Its Top Pick
White Plains, New York, June 21, 2017 — Magnesium supplements are among the most popular supplements, but do you need to take one and, if so, which is best? To find out, ConsumerLab.com reviewed the clinical evidence, and purchased and tested popular magnesium supplements sold in the U.S.
News Release
November 29, 2011
ConsumerLab.com identifies many quality melatonin sleep supplements, but prices range from 4 cents to over $1 for the same dose
White Plains, New York — November 29th, 2011 — Can melatonin help you sleep? "Melatonin supplements may help some people get to sleep sooner, particularly those with chronic sleeping problems, but don't just buy any supplement - they vary significantly in strength, dosage, and cost," ...
News Release
December 30, 2002
Top quality melatonin products identified by ConsumerLab.com — Hormone supplement used to treat sleep disturbances due to jet travel and other causes
WHITE PLAINS, NY — December 30, 2002 — In its latest Product Review, ConsumerLab.com found that 16 of 18 melatonin dietary supplements met their label claims and were free of lead contamination.
Recalls & Warnings
October 02, 2023
Dangerous Ingredients Found in Ten Arthritis and Pain Supplements
On September 29, 2023, the FDA warned consumers that the following arthritis and pain management products contain undeclared drugs such as diclofenac, methocarbamol (a muscle relaxant), and dexamethasone (a corticosteroid), and/or other potentially harmful ingredients.
Recalls & Warnings
August 12, 2024
FDA Warns "Apricot Power" Apricot Seeds Contain Toxic Compound
On May 24, 2024, the FDA warned consumers not to consume three Apricot Power bitter apricot seed products because they were found to contain the toxic compound amygdalin.
Recalls & Warnings
August 31, 2007
FTC Stops Spammers Selling Bogus Hoodia and Human Growth Hormone
On August 23, 2007, the Federal Trade Commission (FTC), announced that spammers must stop sending unwanted and illegal e-mail messages about hoodia weight-loss products and human growth hormone anti-aging products the FTC alleges don't work.
Recalls & Warnings
July 12, 2023
FDA Warns Company for Promoting Kratom Products for Pain, Mood, and More
On July 3, 2023, the FDA issued a warning letter to Sunshine Trading Company, Inc.
Recalls & Warnings
November 09, 2023
Zazzee Naturals Warned for MSM Eye Drop Claims
On October 30, 2023, the FDA issued a Warning Letter to Dexterity Health, LLC DBA Zazzee Naturals following review of the company’s Amazon storefront, which found statements about the company’s Liquid MSM Drops to be drug claims.
Recalls & Warnings
May 01, 2024
FDA Warns Consumers Not to Use Ossos-Sans Supplement for Joints
On April 17, 2024, the FDA warned consumers not to purchase or use Ossos-Sans, a supplement promoted to treat osteoarthritis, osteoporosis, and other conditions, because it contains undeclared drugs, including diclofenac and methocarbamol.
Recalls & Warnings
July 16, 2024
Infla-650 Herbal Dietary Supplement Recalled
On July 16, 2024, Guru Inc. issued a recall of one lot of Infla-650 Herbal Dietary Capsules because they were found to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
July 22, 2024
Umary Hyaluronic Acid Supplement Recalled
On July 15, 2024, SoloVital.
Recalls & Warnings
April 25, 2024
Drug Found in Umary Supplement
On April 18, 2024, CTV News in Canada reported tests suggesting that Umary Hyaluronic Acid Dietary Supplement may be adulterated with the prescription anti-inflammatory drug diclofenac, which may explain side effects that have been reported with its use.
Recalls & Warnings
January 18, 2024
FDA Warns Consumers Not to Use Neptune’s Fix Products
On November 21, 2023, the FDA warned consumers not to purchase or use Neptune’s Fix products because they contain tianeptine. The FDA has received severe adverse event reports after use of these products, including seizure and loss of consciousness.
Recalls & Warnings
April 25, 2024
Yogi Tea Recalled Due to Pesticides
On April 19, 2024, Yogi Tea issued a recall of over 54,800 packs of Yogi Echinacea Immune Support tea after a sample of echinacea tested above acceptable limits for multiple pesticides. No illnesses have been reported to date.
Recalls & Warnings
June 26, 2024
FDA Warns Consumers Not to Use Infla-650 Due to Undeclared Drugs
On June 20, 2024, the FDA warned consumers not to purchase or use Infla-650 after FDA laboratory analysis found them to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
May 18, 2023
EarthLab, Inc. Warned for Promoting Green Tea, Curcumin, Elderberry & More to Treat Stroke, Cancer & Flu
On April 27, 2023, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals, following inspection of the company’s website which found statements about the company’s Green Tea Solid Extract, Curcuma Spp.
Recalls & Warnings
December 29, 2022
EarthLab, Inc. Warned for Promoting Curcumin, Elderberry to Treat Pain & Flu
On November 10, 2022, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals following inspection of the company’s website which found statements about the company’s products to be drug claims because they suggest the products can prevent or treat disease.
Recalls & Warnings
May 05, 2022
Seller of CBD Tinctures, Creams & Pet Products Promoted for Pain, Cancer & More Warned by FDA
On February 11, 2022, the FDA issued a warning letter to Rena’s Organic following a review of the company’s social media and website, which found statements about the company’s 300 mg CBD Full Spectrum Oil Cannabinoid Tincture, 600 mg CBD Full Spectrum Oil Cannabinoid ...
Recalls & Warnings
August 03, 2023
Arsenic Poisoning in U.S. from Ayurvedic Supplements
A 75-year-old woman in California experienced arsenic poisoning after taking Ayurvedic supplements prescribed to her by a holistic energy healer, according to a report published in July in Clinical Case Reports.
Recalls & Warnings
November 28, 2022
Seller of Elderberry, Tea Warned for Claims of Treating Cold, Flu, Cancer
On October 18, 2022, the FDA issued a warning letter to Rosebud’s Ranch and Garden, LLC after inspection of the company’s website and social media found statements about the company’s Domestic Divas – Colds and Flu (Tea), Domestic Divas - No Pain No Gain Tea, Green ...
Recalls & Warnings
May 01, 2023
TruVision Recalls Products Due to Presence of Potentially Dangerous Ingredients
On April 27, 2023, TruVision Health issued a recall of various dietary supplement products because they contain hordenine and/or octodrine/DMHA, compounds that the FDA considers to be “possibly unsafe” and which are not permitted to be sold as dietary supplements.
Recalls & Warnings
September 10, 2014
Seller of Joint Pain Supplement Warned for Drug Claims
On August 24, 2014, the FDA issued a warning letter to CreAgri, Inc., following a website review, which found statements made about the company's products, including Olivenol plus Easeflex, Olivenol plus Essence Capsules, and Olivenol plus Essence Elixer, to be drug claims.
Recalls & Warnings
November 27, 2018
High Levels of Lead Found in Kratom Products
Some kratom products contain levels of lead and nickel that are not considered safe for human consumption, according to tests conducted by the FDA.
Recalls & Warnings
July 06, 2022
FDA Warns Kratom Companies for Arthritis, Pain Relief Claims & More
On January 30, 2022, the FDA issued warning letters to four sellers of kratom products because they were promoted to treat pain relief, opioid withdrawal, blood sugar control, anxiety, and to treat rheumatoid arthritis.
Recalls & Warnings
April 27, 2022
Prescription Drugs Found in Arthritis, Muscle Pain, and Osteoporosis Supplements
On April 20th, 2022, the FDA warned consumers not to use the following ”Artri“ or ”Ortiga” products promoted to treat arthritis, muscle pain, osteoporosis and bone cancer because they have been found to contain undeclared drugs that can have serious adverse ...
Recalls & Warnings
September 17, 2013
Seller of Omega-3 and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On August 29, 2013, the FDA issued a warning letter to Y.S. Health Corp.
Recalls & Warnings
July 11, 2017
FDA Warns Dr. Carolyn Dean of Drug Claims Made for Magnesium, Calcium & Other Mineral Supplements
On June 27, 2017, the FDA issued a Warning Letter to Dr. Carolyn Dean of New Capstone, Inc.
Recalls & Warnings
December 08, 2020
FTC Sends Refund Checks to Consumers of Deceptive Joint Pain Supplement Synovia
On December 1, 2020, the FTC announced it is mailing 13,221 checks totaling nearly $775,000 to consumers who bought Synovia, a supplement intended to treat joint pain and arthritis.
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
Recalls & Warnings
June 16, 2022
Omega 3 Supplements Recalled Due to Undeclared Drug Ingredients
On May 28, 2022, Latin Foods Market issued a voluntary recall of one lot of Artri King Reforzado con Ortiga y Omega 3 tablets after FDA laboratory analysis found the presence of undeclared diclofenac and dexamethasone.
Recalls & Warnings
October 11, 2016
Seller of Glucosamine and Chondroitin Settles FTC Charges of False Advertising
On October 5, 2016, the FTC announced the sellers of the liquid glucosamine and chondroitin supplement Supple (Supple LLC) agreed to settle charges they made false claims about the product.
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Recalls & Warnings
November 20, 2020
FTC Files Complaint Against Two Supplement Companies for Deceptive Marketing
On November 20, 2020, the FTC approved a Part 3 administrative complaint against Health Research Laboratories, LLC, its owner Kramer Duhon, and Whole Body Supplements, LLC for making unverified claims that their products can prevent or treat diseases.
Recalls & Warnings
September 13, 2013
Metagenics Warned For Misbranding of Medical Foods and Drug Claims
On August 13, 2013, the FDA issued a warning letter to Metagenics, Inc.
Recalls & Warnings
November 21, 2017
Health Canada Calls for Stronger Warnings of Liver Risk on Green Tea Extract Products
On November 15, 2017, Health Canada (the Canadian equivalent of the FDA) recommended stronger warning statements about the risk of liver toxicity on labels of supplements containing green tea extract sold in Canada.
Recalls & Warnings
June 26, 2015
Seller of CoQ10, Anxiety Supplements and More Warned for Manufacturing Violations, Drug Claims
On June 6, 2015, the FDA issued a warning letter to Country Doctor Herbals, following a facility inspection which found the company's products, including Herbal Flu Be-Gone, Herbal Multi-Bac, Herbal Nervine, Herbal Pain-A-Way, Herbal Pancreas, Herbal Parasite Cleanse, Herbal Perfect ...
Recalls & Warnings
September 24, 2020
FTC Sends Refund Checks to Consumers of Deceptive Joint Pain Supplement
On September 24, 2020, the FTC announced it is mailing 4,782 checks totaling more than $76,000 to consumers who bought Isoprex, a supplement marketed with deceptive claims that it could treat or cure joint pain, muscle pain, headaches, and arthritis.
Recalls & Warnings
April 17, 2020
Joint Pain Supplement Isoprex Settles Charges of Making False Claims
On April 16, 2020, Renaissance Health Publishing, LLC, agreed to halt their allegedly deceptive advertising claims about their Isoprex supplement that targeted older consumers nationwide after the Federal Trade Commission (FTC) filed a complaint.
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Turmeric, Ashwagandha, Glucosamine, and More
On December 18, 2020, the FDA issued a warning letter to Reddy Naturals following a review of the company's website, which found statements made about the company's products Glycostasis, Glucosamine Curcumin, Turmeric 50, Turmeric Curcumin, Cetyl Curcumin, Ashwagandha, Huruli, ...
Recalls & Warnings
July 10, 2021
FDA Warns Seller of Omega XL
On June 31, 2021 the FDA issued a warning letter to Great Healthworks, Inc. following a review of the company's website, which found statements made about Omega XL and ProbioticXL supplements to be drug claims.
Recalls & Warnings
April 10, 2021
Companies Deceived Consumers About Fish Oil for Liver Disease, Says FTC
On April 1, 2021, the Federal Trade Commission (FTC) announced that fish oil supplement manufacturer BASF SE and its U.S.
Recalls & Warnings
July 24, 2014
Seller of Noni Juice Products Warned for Manufacturing Violations, Drug Claims
On July 14, 2014, the FDA issued a warning to Noni Connection dba Puna Noni, following a facility inspection which found the company's Puna Noni 100% Pure Hawaiian Noni Fruit Capsules to be adulterated because they were prepared, packed, or held under conditions that violate Current Good ...
Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
May 09, 2020
FDA Warns Sellers of Essential Oils, CBD, Vitamins, and More Promoted to Treat Coronavirus
Between May 7 and May 8, 2020, the FDA issued warning letters to seven companies for selling products such as essential oils, CBD, hand sanitizers, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
November 02, 2019
Brain Supplement Claims to Treat Brain Injuries, Alzheimer's Are Deceptive, Says FTC
On November 1, 2019 the FTC filed a lawsuit against Neora LLC (formerly Nerium International) for making false claims that the supplement Neora EHT (formerly called Nerium EHT) can treat concussions and chronic traumatic encephalopathy caused by repetitive brain trauma, Alzheimer's ...
Recalls & Warnings
October 22, 2019
Company Marketing CBD for Use in Infants and Children Gets Strong Warning
On October 10, 2019, the FDA and FTC issued a joint warning letter to Rooted Apothecary LLC for "illegally selling unapproved products containing cannabidiol (CBD) online with unsubstantiated claims" including that the products could treat teething pain and ear aches in infants, autism, ...
Recalls & Warnings
December 05, 2019
Joint Supplement Synovia Was Promoted With Phony Testimonials, Says FTC
A.S. Research, LLC, has agreed to settle Federal Trade Commission (FTC) charges that they deceived consumers with false claims that their dietary supplement Synovia could treat arthritis and alleviate joint pain.
Recalls & Warnings
September 05, 2014
Seller of "Nerve", "Cleanse" and Probiotic Supplements Warned for Drug Claims
On July 30, 2014, the FDA issued a warning letter to Plexus Worldwide, Inc., following a review of the company's website and product labels, which found statements made about Fast Relief, BioCleanse and ProBio5 to be drug claims.
Recalls & Warnings
August 21, 2013
Seller of Vitamin, Tea and MSM Supplements Warned For Drug Claims
August 7, 2013, the FDA issued a warning letter to Vibrant Life Vitamins, following a review of the company's websites which found statements made about Taheebo Life Tea, Life Glow Plus, Super Life Glow, Life Glow Basic, Vibrant Life MSM, Herbal MSM, and Organic Germanium to be drug claims.
Recalls & Warnings
April 28, 2020
Natures CBD Oil Distribution Warned for Joint Pain Claims
On April 20, 2020, the FDA issued a warning letter to Homero Corp DBA Natures CBD Oil Distribution, following an inspection of the company's website which found the company's products, including Natures Pure CBD Oil, CBD Froggies 25mg, CBD Froggies 50mg, CBD Edibles 200mg Frogs, CBD ...
Recalls & Warnings
May 16, 2020
CBD Oil Recalled, Risk of High Lead Exposure
On May 15, 2020, Summitt Labs issued a recall of one batch of KORE ORGANIC Watermelon CBD Oil Tincture due to contamination with lead. A random sample of this product tested by the Florida Department of Agriculture and Consumer Services found it to contain lead levels at 4.7 ppm.
Recalls & Warnings
June 04, 2020
FTC Warns 35 More Companies for Coronavirus Claims
On June 4, 2020, the FTC announced that it sent warning letters to 35 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 15, 2020
FTC Warns 20 More Companies for Coronavirus Claims
On August 14, 2020, the FTC announced that it sent warning letters to 20 companies for selling products such as vitamin C, hydrochloroquine, omega 3, and melatonin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 16, 2020
FDA Warns Four Companies for Unsafe "Homeopathic" Injectables
On June 16, 2020, the FDA issued warning letters to four manufacturers of unapproved injectable drugs labeled as homeopathic.
Recalls & Warnings
February 11, 2020
FTC Sues Two Companies Selling Bone Health and Joint Pain Supplements
On February 11, 2020, the Federal Trade Commission (FTC) announced that it sued two companies to stop them from continuing to make false claims about their bone and joint health products, Ostinol (ZyCal Bioceuticals) and StimTein (Excellent Marketing Results, Inc. (EMR)).
Recalls & Warnings
April 25, 2020
Injectable Curcumin, CBD Are Not Safe, Warns FDA
On April 9, 2020, the FDA issued a warning letter to BIOTA Biosciences LLC following a review of the company's website, which found statements made about some of the company's, products, including Canabidiol (CBD) Complex, Cannabidiol+Curcumin, and Curcumin Complex to be ...
Recalls & Warnings
March 16, 2006
Garden of Life, Maker of Primal Defense, Settles FTC Charges
On March 9, 2006, The Federal Trade Commission (FTC) announced that an operation that marketed dietary supplements sold at Whole Foods Market, GNC, the Vitamin Shoppe, and on the Internet settled FTC charges that they made deceptive advertising claims about their supplements.
Recalls & Warnings
November 07, 2015
Seller of "NaturalDoctor" Vitamin C, Echinacea and More Warned for Manufacturing Violations
On October 16, 2015, the FDA issued a warning letter Sound Healing Arts, PC, dba Grounds for Tea, LLC, following a facility inspection which found the company's products, including NaturalDoctor Vitamins C & K3, NaturalDoctor Goldenseal & Echinacea Plus, NaturalDoctor Centella ...
Recalls & Warnings
July 15, 2014
Vita Springs Health Warned for Manufacturing Violations and Drug Claims
On June 24, 2014, the FDA issued a warning letter to Albert Max Inc.
Recalls & Warnings
August 20, 2019
Seller of Liposomal Curcumin, Vitamin C & Melatonin Warned for Manufacturing Violations
On August 6, 2019, the FDA issued a warning letter to Let's Talk Health, Inc.
Recalls & Warnings
November 24, 2018
Dangerous Drug In Supplements for Pain and Anxiety
On November 20, 2018, the FDA announced it has issued warning letters to two companies for the illegal marketing of products labeled as dietary supplements that contain the opioid-like drug tianeptine.
Recalls & Warnings
September 14, 2016
Seller of Aloe, Prostate and Joint Supplements Warned for Manufacturing Violations
On April 8, 2016, the FDA issued a warning letter to Salud Natural Entrepreneurs, Inc.
Recalls & Warnings
January 17, 2018
Joint and Pain Relief Product Contains Undeclared Drug
On January 17, 2018 Health Canada warned consumers that Linsen Double Caulis Plus, a supplement for joint pain, was found to contain the undeclared drug dexamethasone.
Recalls & Warnings
March 14, 2014
Arthritis and Muscle Pain Supplement Recalled
On March 12, 2014, Pain Free By Nature issued a recall of Reumofan Plus tablets sold on the company's website, www.painfreebynature.com, because it was found to contain the active pharmaceutical ingredients methocarbamol and diclofenac.
Recalls & Warnings
January 16, 2014
Joint Health Supplement Contains Dangerous Drugs, FDA Warns
On January 15, 2014, the FDA warned consumers not to buy or use joint health supplement Pro ArthMax (by Human Science Foundation) because it was found to contain multiple drugs, including diclofenac, ibuprofen, naproxen, indomethacin, nefopam, and chlorzoxazone.
Recalls & Warnings
January 30, 2013
Seller of Omega-3 and Emu Oil Supplements Warned For Drug Claims, Misbranding
On November 19, 2012, the FDA issued a warning letter to Emu Products & Management, Inc. after a review of the company's website found statements that promoted the dietary supplements Multi-Omega Gel Caps, Recovery Gel Caps, ARP Gel Caps and the topical skin products Extreme Cryo Gel, F.A.C.E.
Recalls & Warnings
June 06, 2015
Joint Supplement Contains Drugs, FDA Warns
On June 5, 2015 the FDA warned consumers not to buy or use joint pain supplement Pyrola Advanced Joint Formula because it was found to contain diclofenac and chlorpheniramine.
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
April 02, 2018
NutriZone Kratom Supplements Recalled Due to Salmonella Risk
On March 10, 2018, NutriZone, LLC issued a recall of certain kratom-containing powder products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
November 21, 2014
Herbal Arthritis Supplement Found to Contain Undeclared Drugs
On November 20, 2014, the FDA warned consumers not to purchase or use the herbal dietary supplement Feng Shi Ling because it was found to contain the undeclared drugs diclofenac and indomethacin.
Recalls & Warnings
April 24, 2014
Arthritis Supplement Containing Multiple Drugs Recalled
On April 21, 2014, Nano Well-being Health Inc., issued a voluntary recall of two lots of arthritis supplement Super Arthgold, because it was found to contain the drugs chlorzoxazone, diclofenac and indomethacin.
Recalls & Warnings
September 26, 2014
FDA Warns Seller of Supplements and Chocolate Promoted to Treat Ebola
On September 23, 2014, the FDA issued a warning letter to Natural Solutions Foundation, following a review of the company's websites which found statements made about Silver Sol Nano Silver (also called The Silver Solution) and CBD Organic Dark Chocolate Bars (also called High Potency CBD Hemp Oil) ...
Recalls & Warnings
May 06, 2015
Joint Pain Supplements Found to Contain Diuretics, Antihistamines and Other Undeclared Drugs
On May 4, 2015, the FDA warned consumers not to buy or use the following supplements promoted for joint or back pain because they were found to contain undeclared drugs. Each was identified during an examination of international mail shipments.
Recalls & Warnings
February 13, 2014
FDA Warns Consumers Not To Buy or Use Arthritis Supplement
On February 13, 2014, the FDA warned consumers not to buy or use arthritis supplement Arth-Q because it was found to contain ibuprofen.
Recalls & Warnings
May 10, 2013
Maker of Concentration, Pain, Immune, Cholesterol Supplements Warned For Drug Claims
On April 24, 2013, the FDA issued a warning letter to EuroPharma Co., Inc., following a review of the company's website which found statements made about Calm Kids, CholestCaps, CuraMed 375 mg, CuraMed 750 mg, Curamin, Mental Advantage, Tri-Iodine, and Viragen to be drug claims.
Recalls & Warnings
February 20, 2013
Recall: Arthritis and Muscle Pain Supplement Contains Prescription Drugs
On February 15, 2013, Reumofan Plus USA, LLC and Reumofan USA, LLC announced a recall of Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain, because it was found to contain the active pharmaceutical ingredients methocarbamol, dexamethasone, and diclofenac.
Recalls & Warnings
August 30, 2012
FDA Warns of Medical Claims Made For Numerous Chinese Herbal Products
On August 15, 2012, the FDA warned Dragon Herbs that promotional statements made on the company's website constitute drug claims for twenty herbal products, including CardioPro 2000, Cordyceps, Duanwood Reishi, Gynostemma, Ginseng and Astragalus Combination, Dang Gui and Gelatin, Sweet Relief, ...
Recalls & Warnings
August 29, 2012
FDA Warns Maker of Silver Colloids, Detox and Shell Powder Supplements For Drug Claims, Misbranding
On August 15, 2012, the FDA issued a warning to Healing Ways for statements made on the company's website that constitute drug claims for the company's dietary supplements Silver Colloids, ABC Detox Program and Shell Powder Products.
Recalls & Warnings
July 12, 2013
Herbal Supplement Company Warned For Drug Claims, Manufacturing Violations
On June 18, 2013, the FDA issued a warning letter to Herbs of Light, Inc.
Recalls & Warnings
December 19, 2012
Zicam Nasal Gel Recalled Due to Potential Bacterial Contamination
On December 18, 2012, Matrixx Initiatives issued a voluntary recall of a single lot of Zicam Extreme Congestion Relief nasal gel after routine testing confirmed a sample from this lot was contaminated with Burkholderia cepacia.
Recalls & Warnings
August 09, 2016
Liquid Multis, Vitamin D & More Recalled Due to Potential Bacterial Contamination
On August 8, 2016, PharmaTech, LLC issued a recall of twenty liquid vitamin supplements, sold under various brand names, because they have the potential to be contaminated with Burkholderia cepacia.
Recalls & Warnings
August 19, 2015
Soylent Meal Replacement Powder Reported to Contain Lead and Cadmium
On August 13, 2015, an environmental-health watchdog group, As You Sow, filed a notice of intent to bring legal action against Soylent, a "meal replacement" powder, alleging violation of California's "Prop 65" Safe Drinking Water and Toxic Enforcement Act ...
Recalls & Warnings
February 06, 2016
Seller of Turmeric, Milk Thistle and More Warned for Manufacturing Violations, Drug Claims
On January 15, 2016, the FDA issued a warning letter to Terra Firma Botanicals, Inc.
Recalls & Warnings
January 20, 2016
Seller of Aloe, Moringa Supplements Warned for Manufacturing Violations, Drug Claims
On January 13, 2016, the FDA issued a warning letter to Alkebulan International Services, LLC, following a facility inspection which found the company's products,Aloe Ferox and Moringa Oleifera Capsule to be adulterated because they prepared, packed, or held under conditions that ...
Recalls & Warnings
December 13, 2016
Case of Hemorrhagic Stroke Linked to Redline Energy Drink
A case of hemorrhagic stroke has been linked to the consumption of a single energy drink, according to a report published on October 11, 2016, in The American Journal of Emergency Medicine.
Recalls & Warnings
December 08, 2017
Limbrel Capsules for Osteoarthritis Linked With Potentially Life-Threatening Health Risk, FDA Warns
On December 4, 2017, the FDA urged consumers not to take Limbrel (Primus Pharmaceuticals), a capsule marketed to manage the metabolic processes associated with osteoarthritis, because it has been associated with serious adverse events, including drug-induced liver injury and a lung ...
Recalls & Warnings
June 20, 2003
FTC Charges Marketers of Seasilver with False and Deceptive Claims
On June 19, 2003, The Federal Trade Commission (FTC) and the Food and Drug Administration (FDA) announced coordinated actions against two companies - both charged with promoting the dietary supplement "Seasilver" with unsubstantiated medical claims. The agencies' actions against Seasilver USA, Inc.
Recalls & Warnings
June 04, 2012
FDA Warning on Supplement for Pain Relief
On June 1, 2012, the U.S. FDA warned consumers that Reumofan Plus, marketed as a “natural” dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
Recalls & Warnings
August 22, 2012
FDA Warns of Life-Threatening Side Effects and Withdrawal Symptoms from Two Arthritis, Muscle Pain Supplements
On August 21, 2012, the FDA issued a warning to consumers about the dangers of taking Reumofan Plus and Reumofan Plus Premium, two dietary supplements marketed for arthritis, osteoporosis and muscle pain.
Recalls & Warnings
August 21, 2014
Seller of Joint Supplement Warned for Manufacturing Violations and Drug Claims
On July 17, 2014, the FDA issued a warning letter to Klein Laboratories, Inc.
Recalls & Warnings
November 14, 2014
FDA Warns Consumers Not to Buy or Use Muscle Enhancement Supplement
On November 13, 2014, the FDA advised consumers not to buy or use the muscle enhancement supplement Mayhem (Chaotic-Labz) because it was found to contain the undeclared drugs cyproheptadine and dexamethasone. These drugs can cause serious side effects and can interact with other medications.
Recalls & Warnings
July 04, 2017
FDA Warns Against Company's Unsafe Teething Tablets
On June 20, 2017, the FDA issued a warning letter to Raritan Pharmaceuticals, Inc. following a routine inspection of the facility when it found the company's Homeopathic Infant's Teething Tablets in violation of current good manufacturing practice (CGMP) regulations.
Recalls & Warnings
May 24, 2013
Maker of Neuropathic Pain Product Warned For Drug Claims, Misbranding
On April 11, 2013, the FDA issued a warning letter to Realm Labs, LLC, following a review of the company’s websites which found statements made about its NeuRemedy products to be drug claims.
Recalls & Warnings
August 23, 2013
FDA Warns Consumers of Supplement Containing Prescription Anti-Inflammatory Drug
On August 22, 2013, the FDA warned consumers not to use or purchase the dietary supplement Ortiga, because it contains the prescription drug ingredient, diclofenac.
Recalls & Warnings
June 15, 2003
FTC Alleges "Heartbar" Made Deceptive Claims About Effectiveness
On June 12, 2003, the Federal Trade Commission (FTC) announced that Unither Pharma, Inc. and United Therapeutics Corporation have agreed to settle FTC charges that they made deceptive claims in advertising for their HeartBar product.
Recalls & Warnings
November 28, 2014
Seller of Herbal Extracts Warned for Manufacturing Violations, Drug Claims
On November 3, 2014, the FDA issued a warning letter to Avena Botanicals, Inc.
Recalls & Warnings
September 23, 2014
Maker of Joint and Weight Products Warned for Hidden Drugs, Manufacturing Violations
On September 15, 2014, the FDA issued a warning letter to West Coast Laboratories, Inc., because the company's joint health supplements, Super ArthGold and Pro ArthMax, were found to contain hidden drugs.
Recalls & Warnings
October 03, 2014
Seller of Green Tea, Prostate, Pain Supplements and More Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to AMS Health Sciences, LLC following a facility inspection which found the company's products, including saba ACE, UROPOWER, UROSure, Digest-Eze, Shark Cartilage, Colloidal Silver, and Mobilite to be adulterated because they were prepared, packed, ...
Recalls & Warnings
December 12, 2014
Seller of B-12, Zinc, Echinacea and More Warned for Manufacturing Violations and Drug Claims
On November 14, 2014, the FDA issued a warning letter to Scientific Botanicals Company, Inc.
Recalls & Warnings
August 18, 2017
FDA Warns Consumers Not to Use Certain Liquid Multis, Vitamin D, and Other Supplements Due to Potential Bacterial Contamination
On August 11, 2017, the FDA advised the public not to use any liquid drug or dietary supplement products manufactured by PharmaTech LLC of Davie, Florida, and labeled by Rugby Laboratories, Major Pharmaceuticals, and Leader Brands, because they have the potential to be contaminated with ...
Recalls & Warnings
December 21, 2016
Seller of 5-HTP, Chromium, Curcumin and More Warned for Drug Claims
On December 1, 2016, the FDA issued a warning letter to Aurora Health and Nutrition following a review of the company's website, which found statements made about its products, 5-HTP, Alpha Lipoic Acid, the Argentyn 23 product line, Artecin 90 Vegetarian Capsules, ...
Recalls & Warnings
January 05, 2005
Marketers of Brain-Rejuvinating Supplement Settle FTC Charges of False Claims
On January 5, 2005, the U.S. Federal Trade Commission (FTC) announced that a company that heavily advertised an herbal dietary supplement called "Sagee" to the Chinese-language and Vietnamese-language communities had settled FTC charges of making false and unsubstantiated claims for the product.
Recalls & Warnings
November 02, 2012
Manufacturer of Prostate, Inflammation and Cholesterol Supplements Warned For Drug Claims
On October 15, 2012, the FDA issued a warning letter to Ortho Molecular Products, Inc. for making drug claims about the following dietary supplements: Candicid Forte, Inflamma-bLOX, Lipitrol, Mucosagen, Prostatrol Forte, Resvoxitrol, Paracid Forte, VascuPak HTN and Lithium Orotate.
Recalls & Warnings
April 26, 2013
Seller of Immune, Cholesterol, Liver and Blood Sugar Products Warned For Drug Claims
On March 20, 2013, the FDA issued a warning letter to Birkdale Medicinals LLC, following a review of the company's website which found statements made about Birkdale products, including Immune Response 247, Cholestat, Silymarin 81 and LevelStat, to be drug claims.
Recalls & Warnings
February 21, 2013
Pet Supplement Company Issues Recall Due To Potential Bacterial Contamination
On February 20, 2013, animal supplement company Nutri-Vet, LLC issued a recall of Nutri-Vet and NutriPet Chicken Jerky Products because they may be contaminated with Salmonella.
Recalls & Warnings
May 03, 2013
Homeopathic Company Warned For Drug Claims and Improper Labeling
On March 12, 2013, the FDA issued a warning letter to Natural Medicine Associates, Inc.
Recalls & Warnings
September 10, 2012
Maker of Omega-3, Bone and Brain and Supplements Warned For Drug Claims
On August 27, 2012, the FDA issued a warning letter to PruTect Rx indicating that statements on the company's websites about Omega3PruTect, NeuroPruTect, VitaminD3PruTect and OsteoPruTect constitute drug claims, although the products are not approved drugs.
Recalls & Warnings
November 20, 2013
OxyElite Pro Recall Expanded
On November 19, 2013, USPlabs LLC expanded its recall of OxyElite Pro products to include Raspberry Lemonade OxyELITE Pro Super Thermo Powder (4.6 oz., UPC #094922447494).
Recalls & Warnings
October 09, 2013
FDA Warns Consumers Not to Use OxyElite Pro As It Investigates Link With Hepatitis
On October 8, 2013, the FDA warned consumers not to buy or use USPLabs’ weight loss supplement OxyElite Pro because it has been linked with 24 cases of acute non-viral hepatitis in Hawaii.
Recalls & Warnings
June 27, 2007
Warning Labels to be Required for Green Tea and Black Cohosh Products
On June 26, 2007, the U.S. Pharmacopeia (USP) Dietary Supplements Information Expert Committee (DSI-EC) voted to require cautionary statements on the labels for green tea extracts and black cohosh dietary supplements.
Recalls & Warnings
June 27, 2012
Maker of Pain Relief, Virility, and Idebenone Products Warned of Violations by FDA
On June 19, 2012, the U.S. FDA warned ABCO Laboratories of Fairfield, Caliornia, a contract manufacturer, of violations of FDA regulations for its products IBU-RELIEF 12, Sexual Virility Max, and Idebenone Capsules.
Recalls & Warnings
June 22, 2010
Pet Vitamin Recalled for Salmonella Risk
On June 22, 2010, the FDA reported that United Pet Group, Cincinnati, Ohio is voluntarily recalling all unexpired lots of its PRO-PET ADULT DAILY VITAMIN Supplement tablets for Dogs due to possible Salmonella contamination. This product is not among those included in ConsumerLab.
Recalls & Warnings
November 29, 2016
Seller of Mineral, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On July 1, 2016, the FDA issued a warning letter to BioGenyx-Basic Reset, following a facility inspection which found the company's products, including Ionyte, Aqualyte, Beta Factor, Bee Gold and Primo Java to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
November 28, 2015
Herbal Supplements Linked to Heavy Metal Poisoning
On November 17, 2015, Health Canada (the Canadian equivalent of the FDA) warned that a consumer in Canada who purchased and used herbal supplements from a U.S.-based website was treated for heavy metal poisoning.
Recalls & Warnings
April 17, 2015
Herbal Laxative and "Detox" Kit Containing High Levels of Lead and/or Arsenic Recalled in Canada
On April 15, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced that St. Francis Herb Farm Inc.
Recalls & Warnings
August 06, 2014
Seller of Mineral and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to Mezotrace Corporation following a facility inspection which found the company's products, including Calcium/Magnesium Natural Minerals & Trace Elements, Calcium/Magnesium Natural Minerals & Trace Elements with Vitamin D, and Calcium/Magnesium ...
Recalls & Warnings
September 19, 2018
Seller of Curcumin, Garcinia, Glucosamine and More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to Best Nutrition Products, Inc.
Recalls & Warnings
September 19, 2018
FDA Warns Seller of Shilajit
On September 6, 2018, the FDA issued a warning letter to Adaptive Energy LLC, following an inspection of the company's website, which found statements made about its shilajit product, Purblack were found to be drug claims.
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
August 29, 2017
FDA Warns Seller of Supplements for Allergies, Joint Pain, Bone Health, and More
On August 16, 2017, the FDA issued a warning letter to Total Nutrition, Inc.
Recalls & Warnings
November 25, 2017
Seller of "BounceBack" Joint Supplement and Others Products Warned for Drug Claims
On November 14, 2017, the FDA sent a warning letter to Mannatech Incorporated following a facility inspection that found the products BounceBack, MannaBears, ImmunoSTART, TruSHAPE, and Catalyst to be in violation of the Current Good Manufacturing ...
Recalls & Warnings
December 20, 2017
Seller of Supplements for Pain and Allergies Warned for Manufacturing Violations, Drug Claims
On December 13, 2017, the FDA issued a warning letter to GnuPharma Corporation, following a facility inspection which found a number of the company's products, including Relief capsules, Foundation capsules, Aller-geez capsules, Fit capsules, Aller-Geez Tea, Foundation herbal tea and ...
Recalls & Warnings
February 06, 2018
FDA Links 44 Deaths to Kratom
On February 6, 2018, FDA announced it is now aware of 44 deaths associated with the use of kratom, an herb often promoted for pain relief, energy and for treating opioid withdrawal symptoms. This is an increase from the 36 deaths associated with kratom that the agency reported in November 2017.
Recalls & Warnings
February 02, 2018
Seller of Vitamin D, Omega-3's, Whey Protein & More Warned for Drug Claims
On January 24, 2018 the FDA issued a warning letter to Young Health Products, LLC following a review of the company's website which found promotional statements and testimonials made about the certain products, including Lugol's Solution 2%, Liquid Vitamin D3, Probimune, Flax Seed & Omega ...
Recalls & Warnings
January 21, 2018
Joint Supplement Recalled for Salmonella Risk
On January 21, 2018, Arthri-D, LLC announced a recall of its joint pain supplement, Arthri-D (120 count), due to possible Salmonella contamination.
Recalls & Warnings
July 10, 2018
Blissful Remedies Kratom Recalled Due to Salmonella Risk
On June 30, 2018, Blissful Remedies issued a recall of three kratom products because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
April 21, 2018
NxtGen Botanicals Maeng Da Kratom Recalled
On April 18, 2018, NGB Corp. of West Jordan, Utah issued a recall of NxtGen Botanicals Maeng Da Kratom labeled bottles of encapsulated product because they have the potential to be contaminated with Salmonella. To date, one illness has been reported to the FDA.
Recalls & Warnings
April 09, 2018
Kratom Powders and Capsules Recalled Due to Salmonella Risk
On April 5, 2018, Club 13 issued a recall of certain kratom powders and capsules because they have the potential to be contaminated with Salmonella.





