Product Reviews and Information for Hepatitis (A, B, C)
Search term may appear only in full report available to members. Join now for full access.
Product Review
Milk Thistle Supplements Review
2,800% Difference Found Among Milk Thistle Supplements. Find the Best Milk Thistle Based on Our Tests and See Our Top Picks.

CL Answer
Which vitamins and supplements should be stopped before getting blood work, laboratory tests, or medical imaging?
Find out which vitamins and supplements can interfere with blood, urine or stool tests, and imaginging tests, like bone scans and MRIs. Learn how B vitamins such as biotin, niacin and riboflavin, as well as calcium supplements, St. John’s wort, vitamin C, and others supplements can affect test results, as well as common foods that may interfere with tests.
Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Our Top Picks and Which 5 Failed Testing

Product Review
Multivitamin and Multimineral Supplements Review
Best Multivitamins -- Caution with Gummies

Product Review
Vitamin C Supplements Review
Find the Best Vitamin C Supplements

CL Answer
I thought the B vitamins were all water soluble and did not build up in the body, so you would not build up toxic levels. Am I wrong?
Learn more about water-soluble B vitamins including niacin, B6, B12 and folic acid, and why taking too much of these vitamins can be toxic. See the symptoms of overdose and toxicity from B vitamins. ConsumerLab.com's answer explains.

Product Review
Fruits, Veggies, and Other Greens Supplements Review (Including Spirulina and Chlorella)
Avoid Lead in Greens, Problems with Pills, and Don't Give Up Eating Whole Foods.

Product Review
Farro and Bulgur Review
See How Bob's Red Mill Compares to Trader Joe's

Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

CL Answer
How likely are Americans to be deficient in vitamins or minerals?
Read info based on the CDC about vitamin deficiencies such as vitamin A, B-6, B-12, C, D, and E, folate, and iron in the United States.

CL Answer
Immuno 150: Does It Boost Immunity?
Immuno 150 by Exceptional Health Products is promoted for boosting the immune system, but does it work and is it worth its price? Find out.

CL Answer
Does WEEM Grow Hair and Is It Worth It?
WEEM Hair.Skin.Nails is marketed as a "clinically tested hair health blend.” Find out if it — or its ingredients — works for hair loss and if it's worth the price.

CL Answer
Which supplements reduce hearing loss?
Can supplements such as alpha-lipoic acid, magnesium, N-acetyl-cysteine, folate or vitamins B-12, C and/or E help prevent or improve symptoms among people with hearing loss due to excessive noise or aging? Find out what the clinical evidence shows, and learn whether Advanced Hearing Formula, a supplement marketed for ear health, is likely to help.

Product Review
Baobab Dried Fruit Pulp Review Article
What Are the Benefits of Baobab Dried Fruit Pulp? Find Out What's In Baobab Super Fruit Products, Safety, Side Effects & More.

CL Answer
Supplements for multiple sclerosis (MS): Do any supplements reduce symptoms or relapse?
Find out if vitamins or supplements such as ginkgo, fish oil, biotin, vitamins A, C, E, D, and B12, or selenium reduce symptoms or relapse in people with multiple sclerosis (MS).
CL Answer
Is there a risk of liver toxicity with certain supplements?
Find out if there is risk of liver damage from supplements such as green tea, niacin, red yeast rice and vitamin A.
CL Answer
Is it better to get vitamins from foods or supplements, and are natural vitamins better than synthetic vitamins?
Learn more about the benefits of getting vitamins from food versus supplements, natural versus synthetic vitamins, and more.

CL Answer
Which supplements are important after bariatric surgery (i.e., weight loss or stomach-reducing surgery)? Are there any I should avoid?
Find out which supplements may help after weight loss or stomach reducing surgery, including iron and calcium, and which should be avoided.
CL Answer
Are intravenous (IV) vitamin infusions safe and effective?
Intravenous (IV) vitamin infusions are touted by celebrities and promoted by wellness clinics for numerous conditions. Find out if they work and if they are safe.

CL Answer
I take warfarin (Coumadin), an anticoagulant drug. Are there supplements I should avoid, or be taking, due to this drug?
Find out which supplements should be avoided when taking blood thinners (anticoagulants) like warfarin (Coumadin), including vitamin K, St. John's wort, and curcumin.

CL Answer
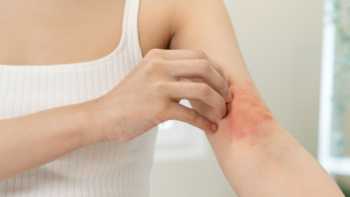
Supplements for Eczema: Some May Help, While Others Don’t
Certain supplements may reduce symptoms of atopic dermatitis (the most common form of eczema), while others don't seem to work. Find out if gamma-linolenic acid, L-histidine, phytoceramides, probiotics, and other supplements may be beneficial.
CL Answer
What is Lipo-flavonoid and does it work for tinnitus or other ear problems?
Lipo-flavonoid Plus info, including ingredients & reliability of claims to improve ear ringing (tinnitus), vertigo, ear aches, ear infection, and Meniere's disease.

CL Answer
What is hydrogen water? Is it beneficial for alertness, athletic performance, arthritis or other conditions, and is it safe?
Find out if hydrogen water has benefits with regard to alertness, side effects of radiotherapy in cancer patients, metabolic syndrome, athletic performance, rheumatoid arthritis, liver disease, and other conditions, and learn if it is safe to use.

CL Answer
Does Provitalize work for weight loss and is it safe?
Find out if taking Provitalize, a probiotic supplement that also contains turmeric extract, moringa leaf extract, curry leaf extract and piperine (BioPerine), relieves joint pain, decreases abdominal fat, or helps with weight loss.

CL Answer
How does Athletic Greens’ AG1 compare to multivitamins and Greens powders? Is AG1 worth it?
ConsumerLab reviews the ingredients in AG1, its safety and possible side effects, and how AG1 compares to other products.

CL Answer
Does Qualia Mind boost memory and mental function and is it worth cost?
Learn about the ingredients in Qualia Mind, whether or not we think the formula works and is safe, and if we think it's worth the cost.

CL Answer
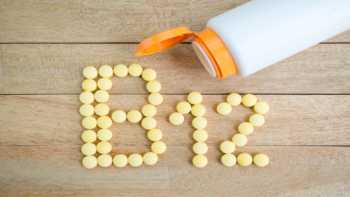
Can taking too much vitamin B-12 be dangerous? The label on my B-complex states it contains 50,000% of the Daily Value!
Find out the daily value for vitamin B-12 and the potential side effects of taking too much B-12. ConsumerLab explains why taking too much vitamin B-12 can be harmful.
CL Answer
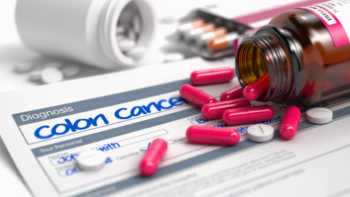
Which supplements help reduce the risk of colorectal cancer?
Find out which supplements may reduce the risk of colorectal cancer, including folate, fiber, selenium, and vitamin D.
CL Answer
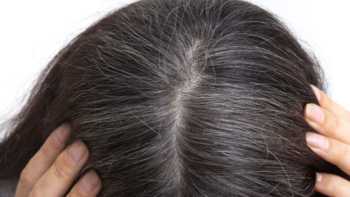
Can any supplements reverse gray hair?
Nearly 75% of adults aged 45 to 65 are affected by gray hair. Learn about what causes gray hair and find out if any supplements can help.
CL Answer
Which vitamins and minerals should be taken together or separately?
Find out the best way to take vitamins and minerals and which vitamins and minerals should be taken together or separately for proper absorption. ConsumerLab answer explains the best way to take vitamins and minerals.
CL Answer
Is it possible to take too much vitamin C?
Find out the daily value of vitamin C, along with possible side effects of excessive vitamin C intake. ConsumerLab explores the topic of proper vitamin C supplement usage, and why taking too much can be harmful.
CL Answer
Desiccated Beef Liver and Beef Organ Supplements: Nutrition & Safety
Description of desiccated beef liver, including what it is used for, its nutrient content, and safety concerns.

CL Answer
Which supplements have been shown to be helpful for autism, and which have not?
Find out which supplements may help reduce symptoms of autism, including multivitamins, probiotics, and melatonin.

CL Answer
Are gummy vitamins better or worse than pills?
Learn more about gummy supplements, including those for multivitamins, vitamins C and D, calcium, and B vitamins.

CL Answer
I take fluoxetine (Prozac), a SSRI drug to treat depression. Are there supplements I should avoid, or be taking, due to this drug?
Learn about Prozac (fluoxetine) drug interactions along with other supplements and SSRIs.
CL Answer
What is TUDCA (tauroursodeoxycholic acid)? Does it have any proven health benefits, and is it safe?
TUDCA information, including its potential health benefits, safety, possible interactions, and cost.
CL Answer
What are "broad-spectrum micronutrient" supplements, and do they have greater health benefits than multivitamin/multimineral supplements?
Learn about "broad-spectrum micronutrients" and how they compare to multivitamin/multimineral supplements.
CL Answer
Do Sugar Bear Hair Vitamins make hair shine, nails stronger, and help both to grow? They taste good but are super expensive.
Info on Sugar Bear Hair vitamins. Do they make hair shine, nails stronger and help hair and nails grow? ConsumerLab.com's answer explains.

CL Answer
Do any supplements reduce the side effects of chemotherapy? Should any be avoided?
Find out if there are any supplements that can help with the side effects of chemotherapy, such as nausea, vomiting, diarrhea, nerve damage and others. ConsumerLab.com's answer explains.

CL Answer
Which vitamins and supplements are good for acne, and are there any that make it worse?
Information about supplements for acne and clearer skin. Plus, find out which vitamins and supplements can make acne worse.

CL Answer
What B-vitamin complex do you recommend for older people?
Vitamin B complex supplement recommendations, benefits and top rated brands for older people and all ages.

CL Answer
What' the Best Way to Take Vitamin B-12? Dosage and Absorption Tips
Discover the optimal dosage and ways to take vitamin B-12, an essential nutrient, for better absorption and effectiveness and learn about possible side effects.
CL Answer
Can taking B vitamin supplements help protect against mosquito bites?
Find out if taking B vitamin supplements can reduce the attraction of mosquitoes to people.

CL Answer
Do supplements help with Parkinson’s disease treatment or prevention?
Learn what has been shown with vitamins D and E, niacin, CoQ10, melatonin, creatine, SAMe, NAC, valerian, CoQ10, and CBD, as well as with coffee and the Mediterranean and MIND diets for treating and/or preventing Parkinson's disease.

CL Answer
Can taking too much vitamin B-6 be dangerous?
Find out if multivitamins contain excessive amounts of B-6, how much B-6 is too much, and symptoms of toxicity and overdose.

CL Answer
Is sublingual vitamin B-12, which is placed under the tongue and allowed to dissolve, really better than the pill form?
Learn the difference between sublingual vitamin B-12, vitamin B-12 pills and more through information including clinical evidence.

CL Answer
Which foods are a good source of B vitamins?
Find out which foods are a good source of B vitamins (like folate and B-12) and when you should take Vitamin B supplements.

CL Answer
Which supplements can help treat anemia? Do any supplements cause a low red blood cell count?
Find out which vitamin and nutrient supplements can help prevent and treat anemia in people who are nutrient deficient and learn which supplements may cause low blood cell counts, especially when used in excess.

CL Answer
When choosing a vitamin C supplement, should you look for one that is "reduced," "L" form (l-ascorbic acid), or "natural"?
Are supplements containing "natural" vitamin C, "reduced" vitamin C, or l-ascorbic acid better than regular vitamin c or ascorbic acid supplements? Find out if these forms are better absorbed or have other advantages, plus, learn about buffered and slow-release forms of vitamin C.

CL Answer
Is Ester-C the best form of vitamin C?
Info on Ester-C Vitamin C, including which form of vitamin C is best, fully-reduced, liposomal and others based on absorption and side effects. ConsumerLab.com's answer explains.

CL Answer
I'm over 50 and looking to take a vitamin B-12 supplement. Many contain a form of vitamin B-12 called cyanocobalamin, yet I read on the Internet that this form is toxic. Should I be concerned?
Looking to see if the cyanocobalamin form of vitamin B-12 is safe? Read research and safety information on ConsumerLab's report.

CL Answer
What is lactoferrin and will it really strengthen my immune system?
Info about lactoferrin supplements, clinical evidence for strengthening the immune system, preventing or treating colds, gut health and treating H.
CL Answer
Can vitamin C reduce the pain of shingles?
Can taking vitamin C for shingles help reduce pain and post-shingles pain, otherwise known as post-herpetic neuralgia? ConsumerLab.com's answer explains.

CL Answer
Can biotin supplements really strengthen nails or improve hair? Can other supplements help?
Learn more about strengthening brittle nails with vitamin B-7 (biotin), based on clinical evidence.

CL Answer
Do any supplements help prevent or improve cataracts?
Find out which supplements may help with cataracts, including selenium and vitamins A, B1, B3, C, and E.

CL Answer
I was told I have a MTHFR gene mutation. Do I need to take methylated forms of B vitamins, such as methylfolate and methylcobalamin?
MTHFR gene mutation can impact B vitamin bioavailability. Learn more about this and which deficiencies are more likely to occur.

CL Answer
Do vitamins, minerals or other supplements lose effectiveness with exposure to high temperatures?
Are vitamins and supplements susceptible to heat? Find out if mail ordering during the summer is safe.

CL Answer
Does taking vitamin C (1,000 mg) deplete copper in the body?
Find out if taking vitamin C supplements can deplete copper levels in the body and cause copper deficiency. ConsumerLab.com's answer explains.

CL Answer
Is P-5-P really better than "regular" vitamin B6?
Learn more about pyridoxal-5-phosphate (P-5-P) and vitamin B6.

CL Answer
Why does my multivitamin make me nauseous? Is there anything that can help?
Find out if ingredients in multivitamins can cause nausea and gastrointestinal discomfort.

CL Answer
Risks of Too Many Vitamins & Supplements
Taking too many vitamin supplements -- or taking too much of a particular supplement -- can have short and long-term adverse effects.

CL Answer
Supplements That May Be Needed When Following a Gluten-Free Diet
Find out if supplements such as folate, vitamin B-12, niacin, thiamin, iron, vitamin D, and/or fiber are needed when following a gluten-free diet. Also learn how to find gluten-free supplements on ConsumerLab.com.

CL Answer
Top 8 vitamins and nutrients for vegetarians and vegans
Find out which 8 supplements may be needed by vegetarians or vegans to meet all their daily nutrient requirements and learn ways to find supplements that do not contain ingredients objectionable to people following vegetarian or vegan diets.

CL Answer
What is Metafolin (5-methylfolate)? I see it in some supplements instead of folic acid. Is it better than folic acid?
Metafolin and Quatrefolic information - compare to folate and folic acid.
CL Answer
Are There Supplements That Help Treat POTS (Postural Orthostatic Tachycardia Syndrome)?
Find out which supplements and lifestyle changes may be helpful for people with Postural Orthostatic Tachycardia Syndrome (POTS).

CL Answer
Do Any Supplements Improve Dry Mouth (Xerostomia) or Make It Worse?
Find out if any supplements, topical formulations (patches, rinses, lozenges, toothpastes, sprays, gels or gums), or lifestyle modifications reduce dry mouths symptoms.

CL Answer
Are there vitamins or supplements that can reduce my risk of breast cancer? Do any increase cancer risk?
Find out if certain vitamins, supplements and foods such as fish oil, olive oil, vitamin C and others, can help reduce the risk of breast cancer. ConsumerLab.com's answer explains.

CL Answer
Does Z-Stack multivitamin work to boost the immune system, and is it safe?
Find out about Z-Stack, including if it can help boost the immune system and if it is safe to use at the suggested dose.

CL Answer
Could taking vitamin C (500 mg), CoQ10, grape seed extract, fish oil, vitamin D3/calcium/magnesium/zinc interfere with taking beta-sitosterol with dinner?
Learn about drug interactions & proper dosages for supplements such as CoQ10, vitamin D & C, fish oil, magnesium, zinc and grape seed extract.

CL Answer
What are the symptoms of vitamin B6 deficiency?
Learn more about symptoms of B6 deficiency, who is more likely to be deficient in vitamin B6 as well as how much vitamin B6 you should be getting. ConsumerLab.com's answer explains.
News Release
February 26, 2025
ConsumerLab Survey Shows 21 Most Popular Vitamins and Supplements
White Plains, New York, February 26, 2025 — A recent survey of more than 8,400 people who regularly use dietary supplements shows that among the 212 most popular supplements, those that experienced the greatest absolute growth in popularity during 2024 were biotin (+9.
Recalls & Warnings
February 22, 2024
Herbalife Shake & Protein Drink Mix, Aloe, Linked With Case of Liver Injury
A 13-year-old boy in Texas developed liver failure and hepatitis-associated aplastic anemia after consuming two Herbalife products and an aloe vera beverage over a period of months, according to a report published on February 12, 2024 in the Journal of Pediatric Gastroenterology and ...
News Release
February 25, 2025
Top-rated Vitamin and Supplement Brands and Merchants for 2025 Based on Consumer Satisfaction
White Plains, New York, February 25, 2025 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 8,400 responses collected in November/December 2024.
News Release
February 24, 2025
Widespread Mislabeling of Nutritional Yeasts Uncovered by ConsumerLab – 90% of Nutritional Yeasts Fail Tests
White Plains, NY, February 24, 2025— Nutritional yeast can add a savory flavor to foods and be a good source of protein, fiber, and niacin. Fortified nutritional yeast can also provide vitamins B-6 and B-12, making it a common addition to vegan and vegetarian dishes.
Recalls & Warnings
August 14, 2023
Liver Injury Linked to "Immune Booster" Supplement Sold on Amazon
A 27-year-old woman in Pennsylvania was admitted to intensive care for drug-induced liver injury after taking Prodigy Life HRP-AID, a supplement sold on Amazon and promoted as an "immune system booster" to reduce the intensity and frequency of cold sore outbreaks, according to a ...
News Release
October 17, 2024
Not All B Vitamin Supplements Contain What They Claim, According to ConsumerLab Tests
White Plains, NY, October 17, 2024 — Recent ConsumerLab tests revealed that 19% of popular B vitamin complexes and individual B vitamin supplements selected for testing contained far less, or far more of one or more B vitamins than claimed on the label.
News Release
February 26, 2024
Latest ConsumerLab Survey Shows Growth in Popularity of Magnesium and Several Smaller Supplements
White Plains, New York, February 26, 2024 — A recent survey of more than 10,000 people who regularly use dietary supplements shows that supplements that experienced the greatest absolute growth in popularity during 2023 were pregnenolone (+7.5 percentage points), magnesium (+4.8 pts), berberine (+4.
News Release
February 25, 2024
Top-rated Vitamin and Supplement Brands and Merchants for 2024 Based on Consumer Satisfaction
White Plains, New York, February 25, 2024 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 10,000 responses collected in November/December 2023.
News Release
December 13, 2023
Best Vitamin C Supplements? ConsumerLab Selects Its Top Picks
White Plains, New York, December 13, 2023 — It’s officially cold season in the U.S., a time when many people may turn to vitamin C supplements to help “boost” their immune system and ward off colds.
News Release
February 25, 2023
Top-rated Vitamin and Supplement Brands and Merchants for 2023 Based on Consumer Satisfaction
White Plains, New York, February 25, 2023 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,600 responses collected in November/December 2022.
Recalls & Warnings
March 23, 2023
FDA Warns Herbal Vitality for Promoting Supplements to Treat Cold & Flu, Kidney Stones & More
On March 7, 2023, the FDA issued a warning letter to Herbal Vitality, Inc.
News Release
February 24, 2023
Probiotics Rise in Popularity as Vitamin C, Melatonin, and Others Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 25, 2023 —A recent survey of 8,600 people who regularly use dietary supplements shows that probiotics (+3.04 percentage points), quercetin (+2.3 pts), and vitamin K (+1.
News Release
November 10, 2022
Best Probiotic Supplements Identified by ConsumerLab Tests
White Plains, New York, November 10, 2022 — Probiotic supplements can be helpful in preventing or treating constipation, diarrhea, flatulence, vaginal infections, and other conditions, but with so many different products on the market, choosing the best one is no easy task.
News Release
June 14, 2022
Best B Complexes, B12, and Other B Vitamin Supplements Revealed by ConsumerLab Tests: Beware of High Doses
White Plains, New York, June 14, 2022 — Recent ConsumerLab tests of over 30 popular vitamin B complexes and supplements revealed that four products contained either much less, or much more, of at least one B vitamin listed on the label.
News Release
February 24, 2022
Consumers Returned to Pre-Pandemic Supplement Usage in 2021, ConsumerLab Survey Reveals
White Plains, New York, February 24, 2022 —A survey of 8,049 people who use dietary supplements shows many supplements that declined in use in 2020 began bouncing back in 2021, such as magnesium (+2.4 percentage points), and CoQ10 (+2.7 pts).
Recalls & Warnings
April 27, 2022
FDA Warns Manufacturer of Topical Antiseptic Products for COVID Claims
On April 19th, 2022, the FDA issued a warning letter to Kleenhanz, LLC following a review of the company’s website and social media which found statements about the company’s Kleenhanz Towelettes topical antiseptic products to be drug claims.
News Release
February 26, 2021
COVID Changed Supplement Popularity in 2020, ConsumerLab Survey Reveals
White Plains, New York, February 26, 2021 — A survey of 9,647 people who use dietary supplements shows that the supplements which experienced the greatest growth in popularity in 2020 were those being promoted to prevent or treat infection with SARS-CoV-2, the coronavirus that causes COVID-19.
News Release
February 12, 2021
Don't Be Surprised by New Labels on Many Vitamins
White Plains, New York, February 12, 2021 — It took almost four years, but more accurate labels on vitamin and mineral supplements are finally here and consumers should not be surprised to see changes, says Tod Cooperman, M.D., president and founder of ConsumerLab.
News Release
June 06, 2020
Best Vitamin C Supplements Revealed by ConsumerLab
White Plains, New York, June 6, 2020 — Due to its role in maintaining immune system health, vitamin C has been promoted to help prevent and treat COVID-19, as well as other viral infections such as colds.
Recalls & Warnings
October 11, 2023
Family Sentenced to Over 12 Years in Prison for Selling Dangerous “Bleach” Miracle Mineral Solution as “COVID Cure”
Four Florida men who distributed the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions have been sentenced to 5 to 12 years in prison for conspiring to defraud the United States.
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
March 18, 2021
Don't Drink Real Water Alkaline Water, FDA Warns After Reports of Liver Illness
On March 16, 2021, the FDA warned consumers not to drink or use Real Water bottled alkaline water while it investigates reports of hepatitis associated with the product.
Recalls & Warnings
January 26, 2023
Allergy Bee Nasal Swabs Contaminated With Illness-Causing Bacteria
On January 18, 2023, the FDA issued a warning letter to Buzzagogo, LLC, after the company’s Allergy Bee Gone for Kids nasal swab products were found to be contaminated with bacteria that have the potential to cause life-threatening illness.
Recalls & Warnings
February 24, 2023
Bountiful Charged with “Review Hijacking,” False Advertising of Nature’s Bounty Supplements on Amazon
On February 16, 2023, the Federal Trade Commission (FTC) charged The Bountiful Company (formerly NBTY), whose brands include Nature’s Bounty and Sundown, with deceptively using Amazon reviews and badges (such as “#1 Best Seller” and “Amazon’s Choice”) from its ...
News Release
February 29, 2020
Collagen and Magnesium Rise in Popularity, as Fish Oil and Curcumin Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 29, 2020 — A recent survey of 9,782 people who use dietary supplements shows that collagen (+ 4.1 percentage points), magnesium (+ 2.3 pts) and CBD (+ 2.
News Release
June 25, 2019
ConsumerLab Tests Reveal Best B Vitamin Supplements -- 19% of B Vitamin Supplements Fail CL's Tests of Quality
White Plains, New York, June 25, 2019 — B vitamins and complexes are among the most popular supplements sold in the U.S. because B vitamins are essential for a wide range of functions in the body.
News Release
February 24, 2019
CBD and Collagen Rise in Popularity, as Probiotics and Coconut Oil Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 24, 2019 — A recent survey of 10,931 people who use dietary supplements shows that CBD oil (+6.3 percentage points), collagen (+3.6 pts), and bone broth (+2.
News Release
December 12, 2018
Best Taurine Supplements for People and Pets Identified by ConsumerLab
White Plains, New York, December 12, 2018 — Supplementing with taurine can treat taurine deficiencies in people and pets which, although uncommon, can occur with unconventional diets, such as grain-free diets for dogs.
News Release
February 24, 2018
Magnesium, Curcumin, and Apple Cider Vinegar Rise in Popularity, While Probiotics Dip and Calcium Continues to Decline in Latest ConsumerLab.com Survey of Supplement Users
White Plains, New York, February 24, 2018 — A recent survey of 11,446 people who use dietary supplements shows that magnesium and curcumin and turmeric have leapt into the top 5 most popular dietary supplements, just behind vitamin D, fish oil and CoQ10.
News Release
September 26, 2017
ConsumerLab.com Explains How to Take Melatonin and Reveals Its Top Supplement Picks
White Plains, New York, September 26, 2017 — Melatonin is now one of the most popular supplements, largely due to its proven ability to trigger sleep.
News Release
March 15, 2017
ConsumerLab.com Reveals Best and Worst Vitamin C Supplements -- 20% Fail ConsumerLab.com Tests of Quality
White Plains, New York, March 15, 2017 — Many people take large doses of vitamin C believing it will improve their health, prevent colds or reduce the risk of disease — but will it really? And when choosing a vitamin C supplement, can consumers be sure it contains what it claims? To ...
Recalls & Warnings
September 28, 2022
FDA Warns Muscle Sports for Joint Health, Immune & Workout Supplement, Vitamin C, Elderberry, and Other Claims
On September 23, 2022, the FDA issued a warning letter to Muscle Sports Products, LLC following inspection of the company’s websites which found statements about company products, including Join Revolution Capsules, IMMUNITY + Powder, Rhino Rampage PUMPED Capsules, Attack Pre-Workout ...
Recalls & Warnings
May 04, 2023
FDA Warns MedoLife for Promoting Homeopathic Products to Treat COVID-19, Cancer
On April 19, 2023, the FDA issued a warning letter to Medolife Rx D/B/A MedoLife Corp., AELIA Inc. and Queanta Inc.
Recalls & Warnings
November 03, 2022
Seller of CBD Warned for COVID-19 Claims
On November 1, 2022, the FDA issued a warning letter to Alternative Health Distribution LLC (d/b/a CannaAid) following a review of the company’s website, which found statements about the company’s cannabidiol (CBD) products to be drug claims.
News Release
February 24, 2017
Vitamin D Supplements Maintain Top Spot in Popularity, as Probiotics Surpass Multivitamins to #4 Behind Fish Oil and CoQ10 in Latest ConsumerLab.com Survey
White Plains, New York, February 24, 2017 — A recent survey of over 9,505 people who use dietary supplements shows the most popular dietary supplement to be vitamin D, followed by fish oil, CoQ10, probiotics, and multivitamins.
News Release
February 23, 2017
ConsumerLab.com Reveals Best and Worst B Vitamin Supplements and Why Current Labels Often Mislead
White Plains, New York, February 24, 2017 — B vitamins and complexes are popular supplements, but labels often mislead, says a new report from ConsumerLab.com which tested, reviewed, and compared 49 supplements from the U.S.
News Release
July 27, 2016
ConsumerLab.com Helps Consumers Make Sense of the FDA's Updated Daily Values (DV) for Vitamins and Minerals
White Plains, New York, July 27, 2016 — Daily Values (DV) for certain vitamins and minerals have been changed by the FDA, as part of the agency's revised rules for nutrient and supplement facts labeling. The changes went into effect on July 26, 2016.
News Release
February 24, 2016
Vitamin D Supplements Jump to Top Spot in Popularity, Surpassing Fish Oil, as Probiotics Rise to #5 Spot Behind CoQ10 and Multivitamins in ConsumerLab.com Survey
White Plains, New York, February 25, 2016 — A recent survey of over 11,000 people who use dietary supplements shows the most popular dietary supplement to be vitamin D, followed by fish oil, CoQ10, multivitamins, and probiotics.
News Release
February 28, 2015
Use of Magnesium and Probiotics Rise, Multivitamins and Weight Loss Supplements Fall, According to ConsumerLab.com Survey
White Plains, New York, February 28, 2015 — A recent survey of over 10,000 people who use dietary supplements shows the most popular dietary supplement to be fish oil, followed by multivitamins, CoQ10, vitamin D, B vitamins, magnesium, calcium, probiotics, and vitamin C.
News Release
December 09, 2014
Round-up of Recent Product Tests by ConsumerLab.com -- Results for B Vitamin Supplements & Energy Drinks, Garlic, NAC (N-acetyl cysteine), Iron and Omega-7 Fatty Acids
White Plains, New York, December 9, 2014 — In recent months, ConsumerLab.com has published test results for a variety of popular supplements, including B vitamins supplements and energy drinks, NAC (N-acetyl cysteine), iron and garlic. In addition, ConsumerLab.
News Release
February 13, 2014
Calcium, Vitamin C, and Fish Oil Supplements Drop in Use, Probiotics Rise According to ConsumerLab.com Survey
White Plains, New York, February 13, 2014 — A recent survey of over 10,000 people who use dietary supplements shows that the use of calcium, vitamin C and fish oil supplements fell during 2013 while the use of probiotics increased.
Recalls & Warnings
December 11, 2020
Seller of "Dr. Hotze's Immune Pak" Products Warned for COVID-19 Claims
Seller of "Dr. Hotze's Immune Pak" Products Warned for Coronavirus Claims
Recalls & Warnings
March 18, 2024
Amazon Customers Who Purchased Nature’s Bounty and Sundown Supplements to Receive Refunds Following “Review Hijacking” Charges
On March 14, 2024, the Federal Trade Commission (FTC) announced it is sending over $527,000 in refunds to consumers who purchased certain Nature’s Bounty and Sundown vitamins and supplements on Amazon, after the parent company Bountiful was charged with deceptive use of reviews to promote the ...
News Release
January 29, 2014
You May Be Getting More Vitamin C Than You Think from Some Supplements, Cautions ConsumerLab.com -- 27% of Vitamin C Supplements Don't Meet Label Claims in Recent Tests --
White Plains, New York, January 29, 2014 — It's cold and flu season, which means many people are reaching for vitamin C, as it may modestly reduce cold symptoms (although it won't prevent a cold).
Recalls & Warnings
November 21, 2024
Douglas Labs Stress-B-Plus Recalled
On October 7, 2024, Nestle Health Science (NHS U.S., LLC) issued a recall of two lots of Douglas Labs Stress-B-Plus because it contains incorrect information on the label.
News Release
January 31, 2013
Use of CoQ10, Digestive Enzymes, Probiotics and B Vitamins on the Rise According to ConsumerLab.com Survey
White Plains, New York — February 1, 2013 — A recent survey of over 10,000 people who take supplements shows that CoQ10, digestive enzymes, probiotics, and B vitamins are the four categories experiencing the most growth over the prior year.
News Release
September 27, 2012
ConsumerLab.com Reveals How Much Caffeine is in Energy Drinks -- Also Finds Some Drinks and Dietary Supplements Don't Contain Claimed Amounts of B Vitamins
White Plains, New York — September 27, 2012 — Energy shots and drinks are promoted to keep you alert and energized, attributing their effects to special formulas often "packed with B vitamins and nutrients to make it last," as a commercial for 5-hour Energy proclaims.
News Release
March 12, 2012
Review of vitamin C supplements by ConsumerLab.com finds many of high quality but a wide range in prices
White Plains, New York — March 8, 2012 — Vitamin C may boost the immune system and is critical for maintaining healthy connective tissue, but do you need to take a supplement and, if so, how do you find one that is right for you and at the best price? ConsumerLab.
News Release
February 05, 2012
Fish oil and multivitamins most popular supplements in ConsumerLab.com survey -- Internet most popular place to shop
WHITE PLAINS, NEW YORK — FEBRUARY 5, 2012 — A survey of over 10,000 savvy consumers of supplements shows the most popular supplements to be fish oil, multivitamins, vitamin D, calcium and CoQ10, in that order.
News Release
October 26, 2010
ConsumerLab.com reports deficiencies in some B-complex supplements and more caffeine than expected in "shot-sized" B vitamin "energy" drinks
White Plains, New York — October 26, 2010 — Tests of B vitamin supplements, including B-complexes and shot-sized energy drinks, revealed problems with the quality of 4 out of 18 products selected for review by independent testing organization ConsumerLab.com.
News Release
March 07, 2008
ConsumerLab.com finds most vitamin C supplements pass quality tests but one short on ingredient and some exceed tolerable levels
WHITE PLAINS, NEW YORK — MARCH 7, 2008 — ConsumerLab.com reported test results today for a wide variety of vitamin C supplements. Vitamin C is the top-selling single vitamin in the U.S., with sales of $863 million in 2006 according to Nutrition Business Journal.
Recalls & Warnings
February 09, 2022
FDA Warns Seller of Colloidal Silver Eye Drops, Copper Products & More
On February 1, 2022, the FDA issued a warning letter to New Earth Healing Essentials, LLC d/b/a 5D Full Disclosure following a review of the company’s website, which found statements made about some of the company's products, including Plasma Colloidal Silver Eyedrops, Gaia’s ...
News Release
September 10, 2007
Tests of B-vitamins by ConsumerLab.com finds several short on folic acid — New report covers B-complexes, thiamin, niacin, B-6, B-12, biotin and folic acid supplements
WHITE PLAINS, NEW YORK — SEPTEMBER 10, 2007 — Half of the B-complex supplements recently selected by ConsumerLab.com for testing were found to lack the total claimed amounts of folic acid, an essential nutrient also known as vitamin B-9.
News Release
March 30, 2005
ConsumerLab.com finds most B-vitamins of high quality but three lacking in ingredients — Test Results for 41 Products Reported Along with Information on Use
WHITE PLAINS, NY — MARCH 30, 2005 — ConsumerLab.com reported test results today for B vitamin supplements that it recently purchased in the U.S. and Canada.
News Release
March 14, 2005
ConsumerLab.com reports on vitamin C supplements — Test Results for 29 Products Reported Along with Information on Use
WHITE PLAINS, NY — MARCH 14, 2005 — ConsumerLab.com reported test results today for a wide variety of vitamin C supplements purchased at retail. Vitamin C is the top-selling single vitamin in the U.S., with sales of $770 million in 2003 according to Nutrition Business Journal.
News Release
July 10, 2002
Pharmavite dietary supplements receive ConsumerLab.com approval — Vitamin E, SAM-e, St. John's Wort, Ginkgo and others merit approved quality products ranking
WHITE PLAINS, NY — July 10, 2002 — ConsumerLab.
News Release
February 08, 2002
Athletic Banned Substances Screening and Certification Program announced by ConsumerLab.com — Tester of supplements to screen for substances banned in Olympics
WHITE PLAINS, NY — February 8, 2002 — ConsumerLab.
Recalls & Warnings
February 12, 2025
Trader Joe’s, Van Camp’s, H-E-B, and Genova Canned Tuna Recalled
On February 7, 2025, Tri-Union Seafoods recalled select canned tunas sold by Trader Joe’s, Van Camp’s, H-E-B, and Genova, due to a manufacturing defect that compromises the integrity of the “easy open” pull tab on the can.
Recalls & Warnings
April 13, 2023
Bountiful to Pay $600,000 to Settle Charges of Amazon “Review Hijacking”
On April 10, 2023, the Federal Trade Commission (FTC) announced it has approved a final consent order against The Bountiful Company (formerly NBTY), whose brands include Nature’s Bounty and Sundown, after the agency charged Bountiful with using deceptive practices to sell its products on ...
News Release
January 08, 2002
ConsumerLab.com finds most B vitamin supplements contain what they claim, but often exceed safe levels — Consumers cautioned to be aware of side effects with high dose products
WHITE PLAINS, NY — January 8, 2002 — ConsumerLab.com announced today that all but one of the twenty-one products analyzed in its B Vitamin Product Review contained the claimed amounts of B vitamins.
News Release
November 20, 2001
ConsumerLab.com finds fish oil supplements free of mercury, but 30% lacking in key ingredient — Test results of omega-3 fatty acid (EPA and DHA) products released today
WHITE PLAINS, NY — November 20, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, released results today of its Product Review of Omega-3 Fatty Acids (EPA & DHA) from Fish/Marine Oils.
News Release
October 03, 2001
Most iron supplements pass ConsumerLab.com testing — Lead contamination and insufficient iron found in some
WHITE PLAINS, NY — October 3, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, released results today of its Product Review of Iron Supplements. Iron deficiency is the most common cause of anemia in the U.S. and worldwide.
News Release
August 15, 2001
Problems and ambiguity among alternative estrogen products reported by ConsumerLab.com — Results of soy and red clover isoflavone product testing released online today
WHITE PLAINS, NY — August 15, 2001 — ConsumerLab.com, an independent evaluator of dietary supplements and nutrition products, today released results of its Phytoestrogen Product Review. The review focused on dietary supplements made from soy and red clover isoflavones.
News Release
May 17, 2000
ConsumerLab.com test of vitamin C products identifies quality control issues and misuse of USP claim.
WHITE PLAINS, NY, May 17, 2000 — Testing of 26 brands of vitamin C dietary supplements indicated that fifteen percent either did not contain all of the claimed ingredient or failed to breakdown as needed for absorption in the body.
News Release
January 31, 2000
Quality of prostate supplements get mixed review in ConsumerLab.com study; Saw palmetto product review published online today.
WHITE PLAINS, NY, January 31, 2000 — Out of 27 major brands of dietary supplements purporting to contain saw palmetto for treating symptoms of prostate enlargement, only 17 appeared to have ingredients similar to those found in published clinical studies.
News Release
January 24, 2000
Majority of dietary supplements sold in U.S. being tested by ConsumerLab.com in 2000; newest results due January 31 for Saw Palmetto.
WHITE PLAINS, NY, January 24, 2000 — As part of its ongoing independent evaluation of health, wellness, and nutrition products sold in the U.S., ConsumerLab.com announced that by year-end it is to have independently tested most of the popular brands of dietary supplements sold in the U.S.
Recalls & Warnings
February 03, 2025
Four Male Enhancement and Energy Supplements Found to Contain Drugs
On January 16, 2025, the FDA issued a Warning Letter to Mihon Corp. d/b/a VitalityVita and Boulla, LLC after FDA analysis found four of the company’s supplements, VitalityXtra, PeakMax, ZapMax, and ZoomMax, to contain undeclared sildenafil.
Recalls & Warnings
May 08, 2021
FDA Warns Seller of Vitamins B12, C, D, and K
On April 7, 2021, the FDA issued a warning letter to Unived Inc following a review of the company's websites, which found statements made about the company's products Unived brand Colox, CalDveg, B12+D3, and D3+K2-7 to be drug claims.
Recalls & Warnings
December 22, 2021
FDA Warns Seller of Liquid Magnesium, B-12, Berberine & More
On December 9, 2021, the FDA issued a warning letter to Wholly Liquid Nutritional Supplements LLC because it found statements on the company's website and social media about its products, including SpiroLaze, BioLaze, LiquiLaurin, VIT-B12, DeStress, and Omega Plus, to be ...
Recalls & Warnings
November 04, 2016
Energy Drink Linked to Case of Acute Hepatitis
A case of acute hepatitis has been linked to daily consumption of energy drinks, according to a report published on November 1, 2016, in BMJ Case Reports.
Recalls & Warnings
August 26, 2014
Maker of Herbal Supplements Warned for Manufacturing Violations and Drug Claims
On August 8, 2014, the FDA issued a warning letter to EnerHealth Botanicals, LLC following a facility inspection which found the company's products, including Parasite Purge Herbal Remedy, Black Walnut Extract, Bladder Cleanse Herbal Extract, Lung Renewal Herbal Remedy, EchinOsha and Daily Immune ...
Recalls & Warnings
July 20, 2023
FTC Files Complaint Against Company Promoting “Sobrenix” Supplements for Alcohol Cravings
On July 19, 2023, the FTC filed a complaint against Rejuvica and the company owners for promoting its Sobrenix supplements, which contain ingredients such as vitamin B6, thiamin, milk thistle, Chanca Piedra, and dandelion root, to reduce and eliminate alcohol cravings and consumption.
Recalls & Warnings
February 06, 2023
FDA Warns Companies Promoting Products to Treat Monkeypox, COVID-19, & Other Viruses
On January 30, 2023, the FDA issued a warning letter to four companies following review of the company websites, which found statements about company products to be drug claims because they were promoted to mitigate, prevent, treat, diagnose, or cure viruses such as monkeypox.
Recalls & Warnings
April 24, 2023
Terry Naturally and EuroMedica B Vitamins Recalled Due to Allergen Risk
On April 21, 2023, EuroPharma, Inc. issued a voluntary recall of 4 lots of its Terry Naturally BioActive Vitamin B 60 count and 3 lots of its EuroMedica Active B Complex 60 count products due to undeclared milk. No illnesses have been reported to date.
Recalls & Warnings
December 01, 2020
Niagen Cannot Be Promoted to Prevent or Treat COVID-19, Warns FDA
On November 17, 2020, the FDA issued warning letters to the sellers of Niagen and Nadovim, supplements that contain different forms of niacin, for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 20, 2019
Seller of Liposomal Curcumin, Vitamin C & Melatonin Warned for Manufacturing Violations
On August 6, 2019, the FDA issued a warning letter to Let's Talk Health, Inc.
Recalls & Warnings
August 15, 2020
FTC Warns 20 More Companies for Coronavirus Claims
On August 14, 2020, the FTC announced that it sent warning letters to 20 companies for selling products such as vitamin C, hydrochloroquine, omega 3, and melatonin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 22, 2022
FDA Warns Seller of Vision, Calcium & Magnesium Supplements and More
On June 14, 2022, the FDA issued a warning letter to New Sun Inc. following inspection of the company’s website which found statements about the company’s products to be drug claims.
Recalls & Warnings
September 05, 2013
Seller of Herbal Supplements and "Tonics" Warned For Manufacturing Violations and Drug Claims
On May 24, 2013, the FDA issued a warning letter to Sundial Herbal Products following a facility inspection which found the company's products, including Woman Back Tonic, Koromantee, Wood and Root Tonic, Arthritis, Asthma, Diabetics, Sundial Ashanti Weight Loss Energy Lifter, Appetite Suppresser, ...
Recalls & Warnings
November 04, 2016
Liver Injuries Linked With Dietary Supplement Use on the Rise
The number of liver injuries associated with dietary supplement use has increased substantially in recent years, according to a recently published study in the journal Hepatology.
Recalls & Warnings
January 16, 2018
Adverse Effects From Energy Drinks Common Among Youth and Young Adults
More than half (55.4%) of young people who have ever consumed an energy drink have experienced at least one adverse reaction, according to study published yesterday in CMAJ Open, a journal of the Canadian Medical Association.
Recalls & Warnings
April 26, 2013
Seller of Immune, Cholesterol, Liver and Blood Sugar Products Warned For Drug Claims
On March 20, 2013, the FDA issued a warning letter to Birkdale Medicinals LLC, following a review of the company's website which found statements made about Birkdale products, including Immune Response 247, Cholestat, Silymarin 81 and LevelStat, to be drug claims.
Recalls & Warnings
December 01, 2018
Seller of 5-HTP, Potassium & More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to The Delano Company, Inc.
Recalls & Warnings
August 18, 2020
Seller of Immune Shot Criminally Charged With Making Coronavirus Claims
On August 10, 2020, prosecutors in Georgia charged Matthew Ryncarz and his company Fusion Health and Vitality, LLC d/b/a/ Pharm Origins with selling the misbranded product Immune Shot.
Recalls & Warnings
April 26, 2021
Family Indicted for Selling Dangerous "Bleach" Miracle Mineral Solution As "COVID" Cure
Four Florida men have been indicted by a Miami federal grand jury on charges of fraudulently marketing and selling the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions, such as cancer, Alzheimer's, diabetes, autism, malaria, ...
Recalls & Warnings
February 13, 2018
American College of Sports Medicine Warns of Energy Drink Dangers
A new statement released by the American College of Sports Medicine (ACSM) highlights the dangers of energy drinks when consumed by children and adolescents, and calls for more studies on the safety and efficacy energy drinks.
Recalls & Warnings
July 21, 2022
UV Light Wands That May Cause Injury, According to the FDA
The FDA recently warned consumers of potential exposure to unsafe levels of ultraviolet-C (UV-C) radiation associated with the use of certain brands of ultraviolet (UV) wands, as found from testing conducted by the agency.
Recalls & Warnings
May 21, 2014
Seller of Gingko and Milk Thistle Warned for Manufacturing Violations and Drug Claims
On April 4, 2014, the FDA issued a warning letter to Xtra Life Natural Systems, Inc.
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Liposomal Vitamin C, Vitamin D & More
On December 21, 2020, the FDA issued a warning letter to Sparrow Health & Performance LLC for selling the products Organic Liposomal Vitamin C, Nanoemulsified D3K2 (also called Liquid Liposomal Vitamin D3 with K2 or Liposomal Vitamin D3) and Immune Support ...
Recalls & Warnings
October 09, 2013
FDA Warns Consumers Not to Use OxyElite Pro As It Investigates Link With Hepatitis
On October 8, 2013, the FDA warned consumers not to buy or use USPLabs’ weight loss supplement OxyElite Pro because it has been linked with 24 cases of acute non-viral hepatitis in Hawaii.
Recalls & Warnings
April 04, 2017
Seller of B Vitamins, Vitamin C, Potassium & More Warned for Manufacturing Violations
On March 24, 2017 the FDA issued a warning letter to The Sanapac Company, Inc.
Recalls & Warnings
August 25, 2009
Hepatitis Associated with Herbal Supplement Containing Artemisinin
The August 14, 2009 edition of the Center for Disease Control's (CDC) Morbidity and Mortality Weekly Report includes case report of hepatitis associated with use of an herbal supplement containing artemisinin.
Recalls & Warnings
November 04, 2015
Green Tea from Tea Bags Linked to Acute Hepatitis
According to a case report published on September 23, 2015 (Lugg, BMJ Case Rep 2015), a 16-year old girl in England developed acute hepatitis in connection with the consumption of green tea.
Recalls & Warnings
August 02, 2013
Vitamin B, Vitamin C and Multimineral Supplements Recalled Due To Anabolic Steroid Risk
On July 31, 2013, Purity First Health Products, Inc.
Recalls & Warnings
September 27, 2016
Seller of B Vitamins, Omega-3s and More Warned for Manufacturing Violations, Drug Claims
On September 15, 2016, the FDA issued a warning letter to Positive Power Nutrition, following a facility inspection which found the company's products, including High Energy C-Complex, Positive Vitality, Positive Performance, Positive CardioGuard, B-Complex 100, Positive Essentials, Positive ...
Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.
Recalls & Warnings
June 02, 2020
Sundial Herbal Ordered to Halt Distribution of Products
On June 1, 2020, a New York federal judge filed an order against two individuals doing business as Sundial Herbal Products to stop the company from distributing its products, which were found to be misbranded and promoted as drugs.
Recalls & Warnings
December 02, 2020
Seller of Vitamin C, Elderberry, Silver, and More Warned for COVID-19 Claims
On November 10, 2020, the FDA issued a warning letter to Sage Woman Herbs, Ltd.
Recalls & Warnings
December 11, 2020
FDA Warns iThrive.health for Promoting Supplements as COVID-19 Treatments
On December 10, 2020, the FDA issued a warning letter to iThrive.health for promoting the sale of various vitamins and supplements on Amazon.com with claims that they can mitigate, prevent, treat, diagnose, or cure COVID-19.
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
March 02, 2021
FDA Warns Seller of Vitamin C, Silver Spray
On March 1, 2021, the FDA issued a warning letter to Ageless Global, LLC following a review of the company's websites for selling Immunoral, Immune Plus, MD Immune Support Spray, and MD CVK-365 Mouth Spray with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
October 30, 2020
Seller of Vitamin C, Vitamin D, Ashwagandha, and More Warned for COVID-19 Claims
On October 23, 2020, the FDA issued a warning letter to Predator Nutrition for selling the products Elixir, Salidroside, Unbreakable, Vitamin C + Bioflavonoids & Rosehip, Vitamin D3, and Ashwagandha with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Flu Immune
On December 21, 2020, the FDA issued a warning letter to Riverstone LLC for selling the products Flu Immune Drops, L-Lysine, Lysine Extra, and Monolaurin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 11, 2020
Colloidal Silver Seller Warned for COVID-19 Claims
On December 2, 2020, the FDA issued a warning letter to Heavenly Natural Products for selling various Carbon 60 and colloidal silver products, including AVOCADO C60 ANTI-VIRAL COMBO — VIRUS PREVENTION (made with C60 WITH AVOCADO and Heavenly Silver) with unsupported ...
Recalls & Warnings
November 28, 2014
Seller of Herbal Extracts Warned for Manufacturing Violations, Drug Claims
On November 3, 2014, the FDA issued a warning letter to Avena Botanicals, Inc.
Recalls & Warnings
May 30, 2017
Seller of Vitamin C, Calcium, Mushroom Supplements and More Warned for Manufacturing Violations
On May 12, 2017 the FDA issued a warning letter to VitaPurity Corporatio.
Recalls & Warnings
December 13, 2016
Case of Hemorrhagic Stroke Linked to Redline Energy Drink
A case of hemorrhagic stroke has been linked to the consumption of a single energy drink, according to a report published on October 11, 2016, in The American Journal of Emergency Medicine.
Recalls & Warnings
July 29, 2017
Increase in Calls to Poison Control Centers About Supplements
The number of calls to poison control centers in the U.S. about dietary supplement exposures increased by almost 50% between 2005 and 2012, according to a study published this week in the Journal of Medical Toxicology.
Recalls & Warnings
September 10, 2012
Maker of Concussion Recovery Supplement Warned For Making Drug Claims
On August 28, 2012, the FDA issued a warning letter to Trinity Sports Group, Inc. after a review of the company's websites found statements about Neuro Impact Concussion Response Formula constituted drug claims not permitted on supplements.
Recalls & Warnings
May 23, 2015
Seller of Magnesium, Calcium, Silver and More Warned for Manufacturing Violations, Drug Claims
On May 8, 2015, the FDA issued a warning letter to Pick and Pay, Inc.
Recalls & Warnings
November 11, 2013
Workout Supplement OxyElite Pro Recalled After Being Linked to Serious Liver Illness
On November 10, 2013, the FDA announced that USPLabs LLC is recalling workout supplements OxyElite Pro Super Thermo capsules, OxyElite Pro Ultra-Intense Thermo capsules and OxyElite Pro Super Thermo Powder because they have been linked to a number of cases of acute non-viral hepatitis, one of which ...
Recalls & Warnings
April 16, 2014
Seller of Aloe, Thyroid and Other Supplements Warned for Manufacturing Violations and Drug Claims
On March 21, 2014, the FDA issued a warning letter to Aloe Man International Corp.
Recalls & Warnings
November 20, 2013
OxyElite Pro Recall Expanded
On November 19, 2013, USPlabs LLC expanded its recall of OxyElite Pro products to include Raspberry Lemonade OxyELITE Pro Super Thermo Powder (4.6 oz., UPC #094922447494).
Recalls & Warnings
June 20, 2020
FTC Warns 30 More Companies for Coronavirus Claims
On June 18, 2020, the FTC announced that it sent warning letters to 30 companies for selling products such as immune system boosters, colloidal silver, vitamin C, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 04, 2020
FTC Warns 35 More Companies for Coronavirus Claims
On June 4, 2020, the FTC announced that it sent warning letters to 35 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
April 14, 2020
FTC Warns Companies Selling Immune "Boosters," Vitamin C and More for Coronavirus Claims
On April 14, 2020, the FTC announced that it has sent warning letters to ten companies for selling products such as immune boosters, silicone facial brushes, air purifiers, and intravenous vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 14, 2020
Sellers of Miracle Mineral Solution Criminally Charged With Making Coronavirus Claims
On July 8, 2020, federal prosecutors charged the sellers of Miracle Mineral Solution, a toxic bleach solution marketed as a cure for coronavirus (COVID-19), with conspiracy to defraud the United States, conspiracy to violate the Federal Food, Drug and Cosmetic Act, and criminal contempt.
Recalls & Warnings
May 23, 2020
FTC Warns 50 More Companies for Coronavirus Claims
On May 21, 2020, the FTC announced that it sent warning letters to 50 companies for selling products such as herbal products, immune system boosters, and vitamin C, with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 19, 2020
FDA Warns Sellers of CBD, Kratom, Vitamin C, and More for Coronavirus Claims
Between May 11 and May 14, 2020, the FDA issued warning letters to five companies for selling products such as vitamin C, immune boosters, kratom, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 06, 2017
Seller of Herbal Supplements Warned for Manufacturing Violations, Drug Claims
On May 25, 2017 the FDA issued a warning letter to Life Rising Corporation, following a facility inspection which found the company's products, including Stomach Regulator, Fang Feng Formula, Skin Regulator, Regulate Liver, Circulation Regulator, Pancreas Support, Lung Regulator, Pure Tea, ...
Recalls & Warnings
March 15, 2013
Maker of Liquid Supplements Warned For Manufacturing Violations and Drug Claims
On January 31, 2013, the FDA issued a warning letter to Liquid Health, Inc.
Recalls & Warnings
July 18, 2018
Maker of Vitamin B12 and Multimineral Supplements Warned by FDA
On July 6, 2018, the FDA issued a warning letter to Aegle Nutrition, LLC, following a facility inspection which found some of the company's products, including Tropical Oasis Ionized Trace Minerals, Tropical Oasis Ultra Methyl B12, and Tropical Oasis Premium Vitamin B12 to be ...
Recalls & Warnings
August 28, 2013
Maker of Sexual Enhancement Supplement Warned for Manufacturing Violations
On July 11, 2013, the FDA issued a warning letter to Precise Nutrition International Inc.
Recalls & Warnings
October 10, 2014
Seller of Multivitamin Warned for Drug Claims
On September 25, 2014, the FDA issued a warning letter to Multimmunity, Inc., following a review of the company's website which found statements made about the dietary supplement Multimmunity to be drug claims.
Recalls & Warnings
June 27, 2017
Seller of Alpha Lipoic Acid, Cinnamon Supplements and More Warned For Manufacturing Violations
On May 1, 2017 the FDA issued a warning letter to Nature's Vision, Inc.
Recalls & Warnings
June 10, 2017
Seller of Prostate, Immune Supplements & More Warned for Manufacturing Violations
On May 25, 2017 the FDA issued a warning letter to The Herbalist, Inc.
Recalls & Warnings
February 07, 2002
Recall of Additional Pepsin-containing Digestive Aids
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its February 6, 2002 Enforcement Report.
Recalls & Warnings
June 23, 2017
FDA Warns Seller of Menopause, Sexual Enhancement, Prostate Supplements and More For Manufacturing Violations
On May 26, 2017 the FDA issued a warning letter to Star Health & Beauty LLC, following a facility inspection which found the company's products, including Nu Essentials Royal Jelly Capsules, NuMan Male Enhancement Capsules, Star's Male Potency Tonic, NuGen HP, She Max HP, and V Max ...
Recalls & Warnings
June 09, 2016
Nature Made Multis and B Complex Recalled Due to Salmonella, Staph Risk
On June 8, 2016 Pharmavite LLC issued a recall of the following Nature Made® multivitamin and B complex supplements because they have the potential to be contaminated with Salmonella or Staphylococcus aureus:
Recalls & Warnings
August 12, 2019
Miracle Mineral Solution Is Dangerous, Warns FDA
On August 12, 2019, the FDA warned consumers not to buy or use Miracle Mineral Solution or other "Miracle" or "Master" solution products containing sodium chlorite because they can dangerous and even life-threatening reactions.
Recalls & Warnings
June 20, 2019
Weight Loss, Muscle & Energy Supplements Linked to Adverse Events in Children and Young Adults
Consumption of dietary supplements sold for weight loss, muscle building, and energy are associated with an increased risk for severe medical events in children and young adults compared to the consumption of vitamins, according to a recent study published in the Journal of the Adolescent ...
Recalls & Warnings
May 09, 2020
FTC Warns 45 More Companies for Coronavirus Claims
On May 7, 2020, the FTC announced that it sent warning letters to 45 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
June 05, 2020
Seller of Silver and Vitamin C Lozenges & More Warned for Coronavirus Claims
On June 1, 2020, the FDA issued a warning letter to Dr.
Recalls & Warnings
February 18, 2020
Seller of CoQ10, Resveratrol and More Warned for Manufacturing Violations
On February 5, 2020, the FDA issued a warning letter to R-Garden LLC, which found the company's Vitamin O, Gamma-Zyme, L.
Recalls & Warnings
November 09, 2019
Biotin Supplements Can Cause False Lab Test Results
On November 5, 2019, the FDA reminded consumers and health care providers that high amounts of biotin (vitamin B-7), found in many supplements, can significantly interfere with certain lab tests and cause incorrect results that may go undetected.
Recalls & Warnings
May 09, 2020
FDA Warns Sellers of Essential Oils, CBD, Vitamins, and More Promoted to Treat Coronavirus
Between May 7 and May 8, 2020, the FDA issued warning letters to seven companies for selling products such as essential oils, CBD, hand sanitizers, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
July 17, 2020
Sellers of Immune Boosters, Vitamin C Warned for Coronavirus Claims
The FDA recently issued warning letters to two companies for selling products such as immune boosters, vitamin C, and zinc with unsupported claims that they can treat COVID-19 (use the links below to read the full warning letter):
Recalls & Warnings
October 27, 2013
Ingredient In Workout Supplements May Be Unsafe, FDA Warns
On October 11, 2013, the FDA directed USP Labs, LLC to cease distribution of workout supplement OxyElite Pro and and muscle-building supplement VERSA-1, which are labeled as containing the ingredient aegeline, or N-[2-hydroxy-2(4-methoxyphenyl) ethyl]-3-phenyl-2-propenamide.
Recalls & Warnings
February 10, 2018
Warning to D-Limonene, Vitamin C Seller
On January 31, 2018 the FDA issued a warning letter to Long Life Unlimited, LLC following a review of the company's website which found promotional statements and testimonials made about products including Balance 600, D-Limonene, Rapha Remedy, Rapha Remedy w/ p73 Wild Oregano, Vitamin C, ...
Recalls & Warnings
September 26, 2012
Maker of Eye Health Supplements Warned for Drug Claims
On September 18, 2012, the FDA issued a warning letter to EyeScience Labs, L.L.C. because statements made about the company's Macular Health Formula, Dry Eye Formula and Diabetic Vision Formula supplements were found to constitute drug claims.
Recalls & Warnings
September 05, 2014
Seller of "Nerve", "Cleanse" and Probiotic Supplements Warned for Drug Claims
On July 30, 2014, the FDA issued a warning letter to Plexus Worldwide, Inc., following a review of the company's website and product labels, which found statements made about Fast Relief, BioCleanse and ProBio5 to be drug claims.
Recalls & Warnings
May 14, 2014
Maker of Mood, Smoking Cessation Supplements and More Warned for Violations, Drug Claims
On April 17, 2014, the FDA issued a warning letter to CDJ Holding, Incorporated, d.b.a.
Recalls & Warnings
November 06, 2014
Seller of Cholesterol, Arthritis Supplements and More Warned for Drug Claims
On October 17, 2014, the FDA issued a warning letter to Vitamins Direct (USA) and Golden Pride, Inc., which distribute Flexezy and Physician's Signature brands of dietary supplements, following a facility inspection which found statements made about certain supplements to be drug claims.
Recalls & Warnings
February 15, 2013
Energy Drink Recalled Due To Bacterial Contamination
On December 21, 2012, NBTY issued a voluntary recall of the energy supplement MET-Rx Extreme Thermo Rage Watermelon 8 fl. oz. (Manufactured by MET-Rx Nutrition, Inc.) due to bacterial contamination.
Recalls & Warnings
December 24, 2013
Muscle Growth Supplement Linked with Liver Failure
On December 23, 2013, the FDA advised consumers to immediately stop using muscle growth supplement Mass Destruction (by Blunt Force Nutrition) because it is labeled as containing at least one synthetic anabolic steroid and has been linked to at least one reported case of liver failure.
Recalls & Warnings
October 22, 2014
Supplements Recalled Years Ago Remain on the Market, Still Contain Hidden Drugs
On October 21, 2014, a report in the Journal of the American Medical Association (JAMA) revealed that almost 10% of the supplements that have been recalled over the past few years are still on the market - and many still contain the hidden drugs which prompted their initial recall.
Recalls & Warnings
May 02, 2017
Seller of Amino Acid Supplements & More Warned for Manufacturing Violations
On April 20, 2017 the FDA issued a warning letter to Naturecom Inc.
Recalls & Warnings
August 01, 2017
FDA Warns Seller of Acai, Garcinia & More for Drug Claims
On July 25, 2017, the FDA issued a warning letter to Absonutrix, following a review of the company's website, www.absonutrix.com, which found statements made about some of its products to be drug claims.
Recalls & Warnings
July 18, 2017
FDA Warns Seller of "Quick Slim with pure Hoodia Gardonii" and "Diabalance Herbal Blood Sugar Balance"
On July 11, 2017, the FDA issued a warning letter to Black Seed Herb, Inc.
Recalls & Warnings
March 10, 2015
Maker of Magnesium, Calcium, Zinc, B Vitamins and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Complete H2O Minerals, following a facility inspection which found the company's products, including Sulfur Concentrate, Copper, Extra Strength Copper, Extra Strength Magnesium, Extra Strength Zinc, Platinum, Gold, Indium, Molybdenum, ...
Recalls & Warnings
December 26, 2012
Seller of Blood Sugar, Depression and Sleep Supplements Warned For Making Drug Claims
On October 15, 2012, the FDA issued a warning letter to BioNeurix Corporation after statements found on the company's website, http://www.pharma-tree.com, were found to promote the dietary supplements Blood Sugar 360, Amoryn, Seredyn, and Mellodyn as drugs.
Recalls & Warnings
April 18, 2013
Cardio, Energy and Sexual Enhancement Supplement Distributor Warned For Manufacturing Violations and Drug Claims
On December 21, 2012, the FDA issued a warning letter to ForMor Inc, dba ForMor International, following a facility inspection which found the company's Cardio Cocktail and Argenix dietary supplements to be adulterated because because they were prepared, packed, or held under conditions that do ...
Recalls & Warnings
August 27, 2013
Immune Supplement Recalled Due To Undeclared Milk
On August 23, 2013, Reaction Nutrition, LLC issued a voluntary recall of immune support supplement LIVE CLINICAL 90 CAPS because it contains undeclared milk.
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
August 28, 2013
Tea and Papaya Supplement Company Warned For Drug Claims
On August 12, 2013, the FDA issued a warning letter to Herbal Papaya, LLC, following a review of the company's website and Facebook page which found statements made about Papaya Seed Extract Capsules, 100% Papaya Leaf (Paw Paw Twig) and Papaya Leaf with Rooibos Tea to be drug claims.
Recalls & Warnings
July 08, 2015
Marketer Prohibited From Promoting Mineral "Cure" For Autism
Kerri Rivera, author of "Healing the Symptoms Known As Autism," has signed an agreement with the Illinois Attorney General which prohibits her from promoting Miracle Mineral Supplement (MMS) as a cure for autism.
Recalls & Warnings
March 15, 2016
Tea Recalled Due to Salmonella Risk
On March 11, 2016 Awareness Corp. issued a recall of its 7.4 ounce container of Boost Tea because it may be contaminated with Salmonella.
Recalls & Warnings
March 19, 2004
Seasilver Stopped from Making Claim to Cure "Over 650" Diseases
On March 17, the Food and Drug Administration (FDA) announced that Seasilver USA, Inc., and Americaloe, Inc.
Recalls & Warnings
February 13, 2013
Manufacturer of Heart, Multivitamin, Cranberry, and Longevity Supplements Warned For Adulteration and Drug Claims
On January 29, 2013, the FDA issued a warning letter to M.D.R. Fitness Corp. following a facility inspection which found violations of good manufacturing practices which cause the company's products to be adulterated.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
October 30, 2014
Seller of Vitamin C, Iron and Detox Supplements Warned for Violations, Drug Claims
On October 16, 2014, the FDA issued a warning letter to Vitalab Co., Inc. (which manufactures and labels supplements for three distributors: V.E. Irons, Inc., Springreen Products, Inc., and Sonne's Organic Foods, Inc.
Recalls & Warnings
January 18, 2002
Recall of Pepsin-containing Digestive Supplements
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its January 16, 2002 Enforcement Report.
Recalls & Warnings
May 17, 2002
Update on Recall of Certain Ultra Slim-Fast with Soy Protein Drinks
In its May 15, 2002 Enforcement Report, the U.S. FDA reported the following Class III Food Recall. A Class III recall is a situation in which use of or exposure to a violative product is not likely to cause adverse health consequences.
Recalls & Warnings
December 05, 2014
Maker of Ginseng, Zinc, Calcium and More Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Long Island Pharmaceuticals, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
May 02, 2016
The Republic of Tea Recalls Turmeric/Ginger Teas Due to Salmonella Risk
On April 29, 2016 The Republic of Tea issued a recall of several Organic Turmeric Ginger teas because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
May 06, 2017
Herbal Teas Recalled Due To Botulism Risk
On May 1, 2017, U.S. Deer Antler Ex. & Imp. of Los Angeles, California, issued a recall of various herbal teas because they have the potential to be contaminated with Clostridium botulinum. This bacterium can cause botulism, a potentially fatal form of food poisoning.
Recalls & Warnings
October 29, 2016
Miracle Mineral Solution Danger
The husband of a woman who died hours after drinking the liquid supplement Miracle Mineral Solution in 2009 believes the supplement caused her death, according a recent ABC News article and feature segment on ABC's 20/20 in October 2016.
Recalls & Warnings
May 06, 2014
Seller of Flaxseed, Ginkgo and More Warned for Manufacturing Violations and Drug Claims
On April 18, 2014, the FDA issued a warning letter to Iowa Select Herbs, LLC, following a facility inspection which found the company's products, including Flax Seed, Holy Basil, Papaya Leaf Extract, and Ginkgo Leaf Extract products, to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
January 07, 2002
Liver Toxicity with Kava
As announced in a December 19, 2001 letter to healthcare professionals, The Food and Drug Administration (FDA) is investigating whether the use of dietary supplements containing kava (also known as kava kava or Piper methysticum) is associated with liver toxicity.
Recalls & Warnings
August 09, 2010
FDA Warns Against "Miracle" Mineral Supplement
On July 30, 2010, The U.S. FDA warned consumers not to consume or use Miracle Mineral Solution, an oral liquid solution also known as "Miracle Mineral Supplement" or "MMS." The product, when used as directed, produces an industrial bleach that can cause serious harm to health.
Recalls & Warnings
May 18, 2012
Caution with Butterbur Indicated by UK Warning
England's Medicines and Healthcare Products Regulatory Agency (MHRA) advised consumers in early 2012 not to take unlicensed butterbur (Petasites hybridus) herbal products due to potential health risks.
Recalls & Warnings
June 20, 2003
FTC Charges Marketers of Seasilver with False and Deceptive Claims
On June 19, 2003, The Federal Trade Commission (FTC) and the Food and Drug Administration (FDA) announced coordinated actions against two companies - both charged with promoting the dietary supplement "Seasilver" with unsubstantiated medical claims. The agencies' actions against Seasilver USA, Inc.
Recalls & Warnings
July 25, 2013
Beware of Dietary Supplements With Claims To Treat Diabetes, FDA Warns
On July 23, 2013, the FDA issued a warning to consumers not to buy or use dietary supplements that are promoted to treat diabetes.
Recalls & Warnings
March 25, 2002
FDA Advises Consumers of Risk of Severe Liver Toxicity with Kava
On March 25, 2002 the U.S. Food and Drug Administration (FDA)issued a Consumer Advisory, as well as a letter to health care providers, advising of the potential risk of severe liver injury associated with the use of kava-containing dietary supplements.
Recalls & Warnings
February 15, 2006
New Zealand Warns of Liver Toxicity from Black Cohosh
On February 9, 2006, New Zealand's Therapeutic Goods Administration (TGA) announced new labelling and consumer information for medicines containing Black cohosh (Cimicifuga racemosa).
Recalls & Warnings
December 22, 2005
Health Canada Warns Consumers Not to Take Chaparral
On December 21, 2005, Health Canada (Canada's health ministry) warned consumers not to ingest the herb chaparral in the form of loose leaves, teas, capsules or bulk herbal products because of the risk of liver and kidney problems.
Recalls & Warnings
May 03, 2013
Maker of Omega-3, Saw Palmetto, St. John’s Wort Supplements And More Warned For Manufacturing Violations and Drug Claims
On March 19, 2013, the FDA issued a warning letter to Sunset Natural Products Inc.
Recalls & Warnings
April 18, 2014
FDA Questions Ingredient in Workout Supplement
On April 4, 2014, the FDA issued a warning letter to Driven Sports Inc., following a label review which found the company's workout supplement, CRAZE, to be adulterated because it contains the new dietary ingredient Dendrobex.
Recalls & Warnings
November 21, 2017
Health Canada Calls for Stronger Warnings of Liver Risk on Green Tea Extract Products
On November 15, 2017, Health Canada (the Canadian equivalent of the FDA) recommended stronger warning statements about the risk of liver toxicity on labels of supplements containing green tea extract sold in Canada.
Recalls & Warnings
January 28, 2014
Emergen-C Settles False Advertising Lawsuit
Alacer Corp., marketers of Emergen-C, has agreed to pay $6.45 million to settle a class action lawsuit which charged the company made false claims that the vitamin C supplement could boost immunity, energy and metabolism.
Recalls & Warnings
March 04, 2011
Recall of "Raw" Vitamin C Supplement Containing Soy
The FDA posted a notice dated March 2, 2011 from Garden of Life, LLC regarding that company's voluntary recall of its Raw Vitamin C supplement because it may contain undeclared soy proteins.
Recalls & Warnings
November 21, 2014
Seller of "Ebola" Supplement Warned for Drug Claims
On November 18, 2014, the FDA issued a warning letter to the seller of dietary supplement Ebola-C, following a review of the company's website, www.ebola-c.com, which found statements made about the product to be drug claims.
Recalls & Warnings
July 28, 2013
B Vitamin Supplement Found To Contain Anabolic Steroids
On July 26, 2013, the FDA warned consumers not to purchase or use Healthy Life Chemistry by Purity First B-50, a vitamin B dietary supplement which was found to contain anabolic steroids, methasterone and dimethazine.
Recalls & Warnings
November 14, 2012
Maker of Vitamin C, Aloe and Sexual Enhancement Supplements Warned for Drug Claims and Misbranding
On October 22, 2012, the FDA issued a warning letter to Health Breakthroughs International, LLC after a review of the company's product labels and website found the dietary supplements Amazing C, MPS-Gold 100 and Power Herbal Formula to be promoted as drugs.
Recalls & Warnings
September 25, 2018
Seller of B Vitamins, Multis, Glucosamine & More Warned for Manufacturing Violations
On August 31, 2018, the FDA issued a warning letter to Independent Nutrition Inc., following a facility inspection which the company's products, including B-50 Complete, Multi-Vitamin & Mineral Complex (a.k.a.
Recalls & Warnings
July 03, 2012
Standard Process Recalls Three Supplements Due to Possible Salmonella Contamination
On June 29, 2012, Standard Process issued a voluntary recall of the company's Cataplex ACP, Cataplex C and Pancreatrophin PMG, after a routine FDA inspection uncovered potential Salmonella contamination in an ingredient found in these three products.
Recalls & Warnings
March 05, 2014
Seller of Aloe and Vitamin C Supplements Warned for Manufacturing Violations
On December 12, 2013, the FDA issued a warning letter to Health Breakthroughs International, following a facility inspection which found the company's products, MPS Gold 100, MPS Gold 3X and Amazing C, to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
July 29, 2016
Ground Turmeric Spice Recalled Due to Lead Contamination
On July 28, 2016, Gel Spice, Inc. issued a voluntary recall one lot of Fresh Finds Ground Turmeric Powder because it was found to contain elevated levels of lead.
Recalls & Warnings
June 13, 2013
Seller of Cardio, Immune, Greens Supplements and More Warned For Drug Claims
On May 29, 2013, the FDA issued a warning letter to dietary supplement company Nature's Answer, following a review a the company's website, www.naturesanswer.
Recalls & Warnings
October 24, 2012
Garlic Supplement Maker Warned For Misbranding and Drug Claims
On October 10, 2012, the FDA issued a letter to dietary supplement manufacturer Garlic Wise because statements made in a brochure about the company’s Alli-C garlic supplement promote the product as a drug. Alli-C was also found to be misbranded due to inadequate directions for use.
Recalls & Warnings
March 08, 2016
Maker of Calcium and Vitamin C Supplements Warned for Manufacturing Violations
On September 17, 2015, the FDA issued a warning letter to Raphah, Inc.
Recalls & Warnings
October 18, 2016
Seller of Sublingual Vitamins Warned for Drug Claims
On August 31, 2016, the FDA issued a warning letter to Bio-Stasis International, Inc., following a review of the company's website which found statements made about its sublingual tablets, ViraPress, Vitamin D-3 and Vitamin B-12. to be drug claims.
Recalls & Warnings
February 28, 2013
Maker of Joint, Mood and B Vitamin Supplements Warned For Manufacturing Violations, Misbranding and Drug Claims
On February 11, 2013, the FDA issued a warning letter to Kreativ Health, Inc.
Recalls & Warnings
January 19, 2018
Flawless Beauty Shut Down, Company's Kits and Products Recalled
On January 19, 2018, Flawless Beauty, LLC issued a recall for all lots of nineteen products sold individually or together as part of its "whitening kits."
Recalls & Warnings
October 29, 2008
Two Vitamin C Supplements Recalled in Canada for Vitamin A Risk
On October 28, 2008, Health Canada warned Canadians, expecially expectant mothers, not to use two vitamin C products sold under the brand names New Roots Herbal Vitamin C8 and Vitazan Professional Vitamin C Advanced Ascorbate.





