Product Reviews and Information for HIV / AIDS
Search term may appear only in full report available to members. Join now for full access.
CL Answer
Do Any Supplements Improve Dry Mouth (Xerostomia) or Make It Worse?
Find out if any supplements, topical formulations (patches, rinses, lozenges, toothpastes, sprays, gels or gums), or lifestyle modifications reduce dry mouths symptoms.

Product Review
DHEA Supplements Review
Choose the Best DHEA Supplement. Beware of Big Differences in Dose and Price.

Product Review
CoQ10 and Ubiquinol Supplements Review
Find the Best CoQ10 and Ubiquinol Supplements and Learn How They Differ

Product Review
CBD Oils, Softgels, Gummies, Creams & Salves Review
See How Much CBD and THC We Found in Products.

CL Answer
Can any vitamins or minerals help prevent or reduce canker sores or cold sores?
Find out which, if any, supplements, including iron, L-lysine, monolaurin, olive leaf extract, vitamin B-12, and zinc, may be beneficial for canker sores or cold sores.
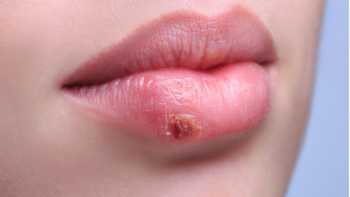
CL Answer
How do over-the-counter (OTC) hearing aids compare to prescription hearing aids?
Non-prescription hearing aid reviews, including MD Hearing Aid, Eargo Hearing Aid, Liberty Hearing Aid, etc. Compare quality & cost with prescription hearing aids.

CL Answer
Do any supplements or foods benefit people with Crohn's disease? Are there any supplements that should be avoided?
Find out the most common vitamin and mineral deficiencies in people with Crohn's disease, including iron, B6, B12, vitamin D, zinc and others, plus evidence for N-acetyl-glucosamine (NAG), fish oil, and curcumin.

CL Answer
Does yacon syrup decrease appetite or help with weight loss? How do I find a quality brand?
Can yacon syrup can help with weight loss? Learn more, including clinical evidence, side effects, and dosage.

CL Answer
Do any supplements or lifestyle changes reduce the symptoms of tinnitus? Is it true that some supplements can cause tinnitus?
Learn which supplements can ease tinnitus, including melatonin and pine bark extract. Understand which may actually cause tinnitus.

Recalls & Warnings
October 11, 2023
Family Sentenced to Over 12 Years in Prison for Selling Dangerous “Bleach” Miracle Mineral Solution as “COVID Cure”
Four Florida men who distributed the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions have been sentenced to 5 to 12 years in prison for conspiring to defraud the United States.
CL Answer
Do any supplements naturally suppress appetite and help with weight loss?
Learn which natural ingredients can suppress appetite and find if these natural appetite suppressants help with weight loss.

CL Answer
Are Hormone Harmony and other Happy Mammoth Supplements Worth Taking?
Are the supplements Hormone Harmony, Hormone Harmony Plus+, Prebiotic Collagen Protein, or Bloat Banisher by Happy Mammoth likely to be beneficial for menopause symptoms or digestive health? Find out.

Recalls & Warnings
April 26, 2021
Family Indicted for Selling Dangerous "Bleach" Miracle Mineral Solution As "COVID" Cure
Four Florida men have been indicted by a Miami federal grand jury on charges of fraudulently marketing and selling the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions, such as cancer, Alzheimer's, diabetes, autism, malaria, ...
Recalls & Warnings
June 02, 2020
Sundial Herbal Ordered to Halt Distribution of Products
On June 1, 2020, a New York federal judge filed an order against two individuals doing business as Sundial Herbal Products to stop the company from distributing its products, which were found to be misbranded and promoted as drugs.
Recalls & Warnings
September 08, 2020
Seller of "Diabetes Supplement" & More Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Nutritional Supplements Corporation Inc following a review of the company's website, which found statements made about some of the company's products, including Vitadone, Diabrex, and Viadevita to be drug claims.
Recalls & Warnings
July 14, 2020
Sellers of Miracle Mineral Solution Criminally Charged With Making Coronavirus Claims
On July 8, 2020, federal prosecutors charged the sellers of Miracle Mineral Solution, a toxic bleach solution marketed as a cure for coronavirus (COVID-19), with conspiracy to defraud the United States, conspiracy to violate the Federal Food, Drug and Cosmetic Act, and criminal contempt.
Recalls & Warnings
August 12, 2019
Miracle Mineral Solution Is Dangerous, Warns FDA
On August 12, 2019, the FDA warned consumers not to buy or use Miracle Mineral Solution or other "Miracle" or "Master" solution products containing sodium chlorite because they can dangerous and even life-threatening reactions.
Recalls & Warnings
January 18, 2005
Supplement Maker to Pay over $3.5 Million to Settle Deceptive Advertising Charges for Immune Dietary Supplement
On January 18, 2005, the Federal Trade Commission (FTC), the Orange County (California) District Attorney, and the California State Attorney General announced that they had reached settlements with Body Wise International, Inc.
Recalls & Warnings
November 03, 2014
Mixing Medications and Dietary Supplements Can Be Dangerous, FDA Warns
On October 27, 2014, the FDA advised consumers to be aware of potential interactions between dietary supplements and over-the-counter (OTC) medications and/or prescription medications.
News Release
February 25, 2024
Top-rated Vitamin and Supplement Brands and Merchants for 2024 Based on Consumer Satisfaction
White Plains, New York, February 25, 2024 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 10,000 responses collected in November/December 2023.
Recalls & Warnings
May 08, 2021
Seller of Immune Bio Green Cell Warned by FDA
On March 30, 2021, the FDA issued a warning letter to Immune & Genetics Protocols, LLC following a review of the company's websites, which found statements made about the company's product Immune Bio Green Cell to be drug claims.
Recalls & Warnings
March 03, 2012
Seller of Cancer Cures Warned by FDA
On February 9, 2012, the U.S. FDA sent a Warning Letter to BioAnue Laboratories, Inc. informing it that its websites at www.tumorx.com, www.cancerx.org, www.hopewelltechnologieslimited.com, and www.vmhe.
Recalls & Warnings
March 06, 2009
"The Truth About Nutrition" Marketers Agree to Pay $3 Million to Settle Charges of Deceptive Advertising of Dietary Supplements and Devices
On March 6, 2008, the Federal Trade Commission (FTC) announced that marketers of dietary supplements and health-related devices have agreed to pay $3 million in consumer redress to settle FTC charges that they deceptively claimed their products treated or prevented a wide variety of serious ...
News Release
August 09, 2023
ConsumerLab Tests Alpha Lipoic Acid Supplements and Selects Top Picks
White Plains, New York, August 9, 2023 — Alpha-lipoic acid supplements may modestly improve insulin sensitivity and blood sugar control, reduce symptoms of peripheral neuropathy, and aid in weight control when combined with a low-calorie diet.
Recalls & Warnings
August 14, 2023
Liver Injury Linked to "Immune Booster" Supplement Sold on Amazon
A 27-year-old woman in Pennsylvania was admitted to intensive care for drug-induced liver injury after taking Prodigy Life HRP-AID, a supplement sold on Amazon and promoted as an "immune system booster" to reduce the intensity and frequency of cold sore outbreaks, according to a ...
News Release
February 25, 2021
Top-rated Vitamin and Supplement Brands and Merchants for 2021 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2021 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,647 responses collected in November 2020.
Recalls & Warnings
December 19, 2020
FDA Finds Unapproved Drugs in Many Weight Loss and Sexual Enhancement Products Sold Online
On December 18, 2020, the FDA warned consumers that certain products promoted for weight loss, body building, sexual enhancement, pain relief, sleep, and other uses because may contain harmful ingredients.
News Release
February 25, 2016
Top-rated Vitamin and Supplement Brands and Merchants for 2016 Based on Consumer Satisfaction
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 11,534 responses collected in November, 2015. Respondents gave ratings for 963 brands and 399 merchants.
News Release
February 25, 2015
Top-rated Vitamin and Supplement Brands and Merchants for 2015 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,329 responses collected in November, 2014. Respondents gave ratings for 1,709 brands and 891 merchants.
News Release
February 12, 2014
Top-rated Vitamin and Supplement Brands and Merchants for 2014 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,326 responses collected in November, 2013. Respondents gave ratings for 1,639 brands and 788 merchants.
News Release
February 01, 2013
Top-rated Vitamin and Supplement Brands and Merchants for 2013 Based on Consumer Satisfaction
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,862 responses collected in November, 2012. Respondents gave ratings for 1,438 brands and 851 merchants.
News Release
December 12, 2006
Probiotic supplements grow in popularity but viable bacteria missing in many — ConsumerLab.com cautions consumers to select probiotics carefully and store them properly
WHITE PLAINS, NEW YORK — DECEMBER 12, 2006 — Sales of supplements containing beneficial organisms (probiotics) have grown rapidly, but a new test report from ConsumerLab.com shows that 44% contained fewer viable organisms than claimed or generally known to be effective.
News Release
October 20, 2006
ConsumerLab.com report on CoQ10 supplements finds quality high but large range in suggested dosage
WHITE PLAINS, NEW YORK — OCTOBER 20, 2006 — New test results from ConsumerLab.com for CoQ10 supplements show that all of the products selected contained amounts of ingredient consistent with their labels.
News Release
May 10, 2005
ConsumerLab.com begins publishing test reports on Japanese vitamins and supplements — Review of CoQ10 products Now Available Online in Japanese at ConsumerLab.jp. More Reports Scheduled
— Review of CoQ10 Products Now Available Online in Japanese at ConsumerLab.jp. More Reports Scheduled — WHITE PLAINS, NY — MAY 10, 2005 — ConsumerLab.
News Release
November 21, 2000
ConsumerLab.com tests popular supplement for heart failure; CoQ10 test results released online today
WHITE PLAINS, NY — November 21, 2000 — ConsumerLab.com today released results of its 10th Product Review, focusing on dietary supplements containing coenzyme Q10 (CoQ10).
Recalls & Warnings
April 27, 2022
FDA Warns Manufacturer of Topical Antiseptic Products for COVID Claims
On April 19th, 2022, the FDA issued a warning letter to Kleenhanz, LLC following a review of the company’s website and social media which found statements about the company’s Kleenhanz Towelettes topical antiseptic products to be drug claims.
Recalls & Warnings
February 06, 2023
FDA Warns Companies Promoting Products to Treat Monkeypox, COVID-19, & Other Viruses
On January 30, 2023, the FDA issued a warning letter to four companies following review of the company websites, which found statements about company products to be drug claims because they were promoted to mitigate, prevent, treat, diagnose, or cure viruses such as monkeypox.
Recalls & Warnings
July 22, 2022
FDA Warns Seller of Vision, Calcium & Magnesium Supplements and More
On June 14, 2022, the FDA issued a warning letter to New Sun Inc. following inspection of the company’s website which found statements about the company’s products to be drug claims.
Recalls & Warnings
February 17, 2021
FDA Warns Dr. Paul's Lab for COVID-19 Claims
On February 16, 2021, the FDA issued a warning letter to Dr. Paul's Lab following a review of the company's website by the FDA and Federal Trade Commission (FTC) for selling COVID-Aid Tincture with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
August 25, 2022
Oregon’s Wild Harvest Warned by FDA for Manufacturing Violations
On July 8, 2022, the FDA issued a warning letter to Oregon’s Wild Harvest, Inc.
Recalls & Warnings
May 03, 2011
FDA Warns: Beware of Bogus STD Products
On May 3, 2011, the U.S. Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) announced a joint effort to remove products from the market that make unproven claims to treat, cure, and prevent sexually transmitted diseases (STDs).
Recalls & Warnings
June 07, 2013
Maker of Colloidal Silver and Mushroom Extract Supplements Warned For Manufacturing Violations and Drug Claims
On May 2, 2013, the FDA issued a warning letter to Earthborn Products, Inc.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Turmeric, Ashwagandha, Glucosamine, and More
On December 18, 2020, the FDA issued a warning letter to Reddy Naturals following a review of the company's website, which found statements made about the company's products Glycostasis, Glucosamine Curcumin, Turmeric 50, Turmeric Curcumin, Cetyl Curcumin, Ashwagandha, Huruli, ...
Recalls & Warnings
June 05, 2020
LifeHealth Science Warned for Manufacturing Violations
On May 15, 2020, the FDA issued a warning letter to LifeHealth Science, which found the company's ORËÁ product to be adulterated because it was prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
July 08, 2015
Marketer Prohibited From Promoting Mineral "Cure" For Autism
Kerri Rivera, author of "Healing the Symptoms Known As Autism," has signed an agreement with the Illinois Attorney General which prohibits her from promoting Miracle Mineral Supplement (MMS) as a cure for autism.
Recalls & Warnings
August 18, 2006
Restitution Program for Purchases of Lane Labs' Products
On August 17, 2006 the Food and Drug Administration (FDA) announced that it was notifying consumers of a restitution (refund) program for purchasers of three of Lane Labs-USA, Inc.'s products. The products are BeneFin, MGN-3 and SkinAnswer.
Recalls & Warnings
June 05, 2020
Seller of CBD, Sleep Aids, and Cold & Flu Products Warned for Manufacturing Violations
On April 28, 2020, the FDA issued a warning letter to The Dragontree Apothecary LLC, which found the company's Sleep Support, Anxiety Relief, Cold & Flu Relief, and Inflammation Relief to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
April 25, 2020
Injectable Curcumin, CBD Are Not Safe, Warns FDA
On April 9, 2020, the FDA issued a warning letter to BIOTA Biosciences LLC following a review of the company's website, which found statements made about some of the company's, products, including Canabidiol (CBD) Complex, Cannabidiol+Curcumin, and Curcumin Complex to be ...
Recalls & Warnings
April 11, 2020
FDA Warns Sellers of CBD, Colloidal Silver & Natural Remedies Promoted to Treat Coronavirus
Between April 7 and April 9, 2020, the FDA issued warning letters to five companies for selling products such as CBD, colloidal silver, and natural treatments with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
January 18, 2002
Recall of Pepsin-containing Digestive Supplements
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its January 16, 2002 Enforcement Report.
Recalls & Warnings
August 26, 2014
Maker of Herbal Supplements Warned for Manufacturing Violations and Drug Claims
On August 8, 2014, the FDA issued a warning letter to EnerHealth Botanicals, LLC following a facility inspection which found the company's products, including Parasite Purge Herbal Remedy, Black Walnut Extract, Bladder Cleanse Herbal Extract, Lung Renewal Herbal Remedy, EchinOsha and Daily Immune ...
Recalls & Warnings
August 09, 2010
FDA Warns Against "Miracle" Mineral Supplement
On July 30, 2010, The U.S. FDA warned consumers not to consume or use Miracle Mineral Solution, an oral liquid solution also known as "Miracle Mineral Supplement" or "MMS." The product, when used as directed, produces an industrial bleach that can cause serious harm to health.
Recalls & Warnings
August 30, 2012
FDA Warns of Medical Claims Made For Numerous Chinese Herbal Products
On August 15, 2012, the FDA warned Dragon Herbs that promotional statements made on the company's website constitute drug claims for twenty herbal products, including CardioPro 2000, Cordyceps, Duanwood Reishi, Gynostemma, Ginseng and Astragalus Combination, Dang Gui and Gelatin, Sweet Relief, ...
Recalls & Warnings
August 28, 2013
Tea and Papaya Supplement Company Warned For Drug Claims
On August 12, 2013, the FDA issued a warning letter to Herbal Papaya, LLC, following a review of the company's website and Facebook page which found statements made about Papaya Seed Extract Capsules, 100% Papaya Leaf (Paw Paw Twig) and Papaya Leaf with Rooibos Tea to be drug claims.
Recalls & Warnings
August 09, 2007
FDA Warning on Red Yeast Rice Products
On August 9, 2007, the U.S. Food and Drug Administration (FDA) warned consumers not to buy or eat three red yeast rice products promoted and sold on Web sites. The products may contain an unauthorized drug that could be harmful to health.
Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
Recalls & Warnings
November 02, 2012
Maker of Joint Health, Blood Sugar, Prostate, Ginkgo Supplements and More Warned For Drug Claims, Misbranding and Adulteration
On September 10, 2012, the FDA issued a warning letter to Global Source Management & Consulting for making drug claims about the following dietary supplements: Scientific Joint Program capsules, Alpha Lipoic Acid, Healthy Blood Sugar Diabetic Support, Dr.
Recalls & Warnings
December 23, 2017
Seller of Meal Replacement, Protein Drinks, Cranberry and More Warned for Manufacturing Violations
On July 6, 2017, the FDA issued a warning letter to Professional Botanicals, Inc.
Recalls & Warnings
January 27, 2003
FTC Charges Dr. Clark and Related Companies with Making Unsubstantiated Health Claims
The Federal Trade Commission has charged a Switzerland-based company and its U.S. counterpart with making numerous unsubstantiated efficacy claims for a variety of dietary supplements and devices that they sell on the Internet.
Recalls & Warnings
October 17, 2012
Herbal Supplement Company Warned For Manufacturing Violations and Drug Claims
On October 2, 2012, the FDA issued a warning letter to Amazing Herbs Nutraceuticals following a facility inspection which found violations of Current Good Manufacturing Practices (CGMPs) for dietary supplements.
Recalls & Warnings
August 08, 2013
Sleep Supplement Recalled Due To Undeclared Drugs
On August 7, 2013, Health and Beyond LLC issued a voluntary recall of dietary supplement Tranquility because it was found to contain traces of undeclared drugs, Doxepin and Chlorpromazine.
Recalls & Warnings
February 01, 2013
Lactase Enzyme Supplement Recalled
On November 13, 2012, Cardinal Health issued a voluntary recall of specific lots of Dairy Aid Lactase Enzyme Supplement 3,000 FCC Unit caplets because they are incorrectly labeled as 9,000 FCC Unit caplets.
Recalls & Warnings
January 04, 2012
Seller of "HCG Drops" for Weight Loss Warned by FDA and FTC of Violations
On December 21, 2011, the U.S. FDA and FTC sent a joint Warning Letter to hCG Drops LLC regarding its marketing of Slim Diet Drops. According to the letter, the product is considered an unapproved drug because it is promoted for drug-like uses on the company's website, including the following:
Recalls & Warnings
June 20, 2003
FTC Charges Marketers of Seasilver with False and Deceptive Claims
On June 19, 2003, The Federal Trade Commission (FTC) and the Food and Drug Administration (FDA) announced coordinated actions against two companies - both charged with promoting the dietary supplement "Seasilver" with unsubstantiated medical claims. The agencies' actions against Seasilver USA, Inc.
Recalls & Warnings
July 24, 2006
Marketers of Seasilver Ordered to Pay Almost $120 Million
On July 24, 2006, the Federal Trade Commission (FTC) announced that the marketers of Seasilver, an alleged phony cure-all, have been ordered to pay almost $120 million for failing to comply with an earlier order requiring them to pay $3 million in consumer redress.
Recalls & Warnings
February 07, 2002
Recall of Additional Pepsin-containing Digestive Aids
The U.S. Food and Drug Administration (FDA) released the following Class I recall information in its February 6, 2002 Enforcement Report.
Recalls & Warnings
September 19, 2018
FDA Warns Seller of Shilajit
On September 6, 2018, the FDA issued a warning letter to Adaptive Energy LLC, following an inspection of the company's website, which found statements made about its shilajit product, Purblack were found to be drug claims.
Recalls & Warnings
March 13, 2018
FDA Warns Seller of Milk Thistle, Chromium, Joint Supplements & More for Manufacturing Violations
On March 9, 2018, the FDA issued a warning letter to Carol Bond Health Foods following a facility inspection which found a number of the company's products, including Dolomite, Lipo Fat Fighter, ThistleX, Injuv, and Vinpocentine, to be ...
Recalls & Warnings
February 01, 2018
Seller of Supplements for Opiate Withdrawal Warned for Drug Claims
On January 11, 2018, the FDA sent warning letters to ten sellers of supplements promoted to treat opiate withdrawal following reviews of the companies' websites and social media which found statements and testimonials made about the products to be drug claims.
Recalls & Warnings
June 07, 2011
FDA Seizes Elderberry Juice Concentrate Due to Unproven Claims
On June 3, 2011, The U.S. FDA announced that, at its request, U.S. Marshals seized elderberry juice products that have been distributed by Wyldewood Cellars Inc., based in Peck, Kan., because the products are unapproved and misbranded drugs.
Recalls & Warnings
April 22, 2008
Court Upholds Order for Seasilver Marketers to Pay $120 Million
On April 16, 2008, the Federal Trade Commission (FTC) announced that the U.S.
Recalls & Warnings
August 27, 2013
FTC Refunds Consumers of Children's Vitamins as Part of Deceptive Advertising Settlement
On August 8, 2013, the Federal Trade Commission (FTC) announced it has mailed 10,144 refund checks to consumers who purchased Disney or Marvel Hero -themed children's vitamins, including Disney Princesses, Winnie the Pooh, Finding Nemo, and Spider-Man varieties, between May 1, 2008 and September ...
Recalls & Warnings
May 03, 2013
Homeopathic Company Warned For Drug Claims and Improper Labeling
On March 12, 2013, the FDA issued a warning letter to Natural Medicine Associates, Inc.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
October 13, 2017
Hi-Tech To Pay Over 40 Million to Settle FTC Charges of False Claims
On October 11, 2017, a U.S. District Judge ruled that Hi-Tech Pharmaceuticals Inc.
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
April 04, 2017
Seller of B Vitamins, Vitamin C, Potassium & More Warned for Manufacturing Violations
On March 24, 2017 the FDA issued a warning letter to The Sanapac Company, Inc.
Recalls & Warnings
October 29, 2016
Miracle Mineral Solution Danger
The husband of a woman who died hours after drinking the liquid supplement Miracle Mineral Solution in 2009 believes the supplement caused her death, according a recent ABC News article and feature segment on ABC's 20/20 in October 2016.
Recalls & Warnings
March 29, 2016
Maker of Bone Health, Prostate, Cholesterol Supplements and More Warned for Drug Claims
On March 17, 2016, the FDA issued a warning letter to Rx Vitamins, Inc., following a facility inspection which found the company's products, includingTestost-Rx, DB-7, The Bone Density Formula, NaturLo Cholesterol, ThyRx-7 and Arth-9 to be misbranded.
Recalls & Warnings
December 29, 2015
Maker of Vitamin K, Vitamin A & More Warned for Manufacturing Violations, Drug Claims
On December 10 2015, the FDA issued a warning letter to Dherbs Health Emporium, Inc.
Recalls & Warnings
November 18, 2015
Seller of "Hangover" Supplement Warned for Manufacturing Violations & Drug Claims
On September 17, 2015, the FDA issued a warning letter to Life Support Development Ltd, following a facility inspection which found the company's product, including Life Support Hangover Relief to be adulterated because they prepared, packed, or held under conditions that violate ...
Recalls & Warnings
August 06, 2014
Seller of Mineral and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to Mezotrace Corporation following a facility inspection which found the company's products, including Calcium/Magnesium Natural Minerals & Trace Elements, Calcium/Magnesium Natural Minerals & Trace Elements with Vitamin D, and Calcium/Magnesium ...
Recalls & Warnings
June 12, 2014
Marketers of Memory Supplement Settle FTC Charges of Deceptive Claims
Martek Biosciences Corporation and i-Health Inc., marketers of BrainStrong Adult, have agreed to settle Federal Trade Commission (FTC) charges that claims the supplement could improve adult memory and prevent cognitive decline were deceptive, and not supported by sufficient clinical evidence.
Recalls & Warnings
February 13, 2014
Maker of Cholesterol and Workout Supplements Warned For Manufacturing Violations, Drug Claims and Unapproved Ingredient
On January 31, 2014, the FDA issued a warning letter to Exclusive Supplements Inc.
Recalls & Warnings
December 12, 2014
Marketers of HCG Settle FTC Charges of Deceptive Weight Loss Claims
Marketers of HCG Platinum drops have agreed to pay $1 million to settle Federal Trade Commission (FTC) charges that claims the drops could cause rapid and substantial weight loss were deceptive and not supported by scientific evidence.





