Reviews and Information for Manuka Honey
Manuka Honey
Read our review to find the best manuka honey for quality, value, and taste, based on ConsumerLab's tests. Also learn whether manuka honey reduces sore throat or acid reflux, or improves wound healing, or has other health benefits, what to look for on manuka honey labels, and the safety of manuka honey.
Manuka Honey ReviewSearch term may appear only in full report available to members. Join now for full access.
News Release
May 12, 2025
Best & Worst Manuka Honey Identified by ConsumerLab Tests
White Plains, NY, May 12, 2025 -- Manuka honey is often promoted for wound healing, and for soothing sore throats, cough, acid reflux, and other conditions, due to its high concentration of the antibacterial compound methylglyoxal (MGO), and other compounds derived from the manuka bush.
Recalls & Warnings
June 18, 2025
Comvita Manuka Honey + Reishi and Lion's Mane Products Recalled
On May 23, 2025, Comvita USA INC recalled certain Comvita branded Manuka Honey + Reishi, Comvita branded Manuka Honey + Cordyceps for Energy, and Comvita branded Manuka Honey + Lion's Mane for Focus products due to a leaking induction seal on the packaging.
CL Answer
Do Any Supplements Improve Dry Mouth (Xerostomia) or Make It Worse?
Find out if any supplements, topical formulations (patches, rinses, lozenges, toothpastes, sprays, gels or gums), or lifestyle modifications reduce dry mouths symptoms.

CL Answer
Do any supplements help prevent or treat a cold?
Are there supplements that can help prevent or treat cold symptoms? Learn more about zinc, probiotics, vitamin C and more.

CL Answer
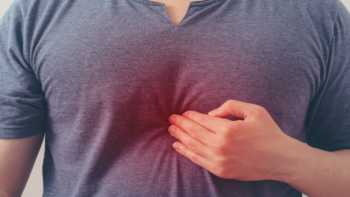
Which supplements, foods or diet and lifestyle changes help relieve acid reflux (heartburn), and which worsen it?
Find out which supplements and foods can help relieve acid reflux, and which can make it worse.
CL Answer
Which supplements are best for seasonal allergies?
Find out which supplements are best for allergies and can help reduce seasonal allergy symptoms, including bromelain, curcumin, vitamin D, and CLA.

Recalls & Warnings
February 28, 2024
Microbial Contamination Found in LightEyez MSM Eye Drops
On February 15, 2024, the FDA issued a Warning Letter to LightEyez Limited after testing by the agency found the company’s LightEyez.com MSM Eye Drops V2.0 — Eye Repair (MSM Eye Repair Drops) to have microbial contamination in samples of the drops.
CL Answer
Sugar Substitutes: Pros, Cons, and Best Choices
Learn about the pros and cons of using sugar substitutes and artificial sweeteners in place of table sugar. Sweeteners discussed include stevia, monk fruit, and other high-intensity sweeteners; xylitol, erythritol, allulose and other low-calorie sweeteners; and agave syrup, black sugar, coconut sugar, honey, molasses and other sugar alternatives.

Product Review
Elderberry Supplements Review
Find the Best Elderberry Supplement. Tests and Reviews of Popular Elderberry Supplements & CL's Top Picks.

CL Answer
8 Supplements That May Help Treat Peptic Ulcers – And Some to Avoid
Peptic ulcers caused by H. pylori infection are typically treated with antibiotics, but certain supplements may also help. Find out which 8 supplements might be beneficial - and which should be avoided.

CL Answer
Bee pollen: Purported health benefits and possible side effects
Bee pollen is promoted for numerous health conditions, including supporting immune health and boosting energy. Find out if it works and if it's safe.

CL Answer
Is ingesting colloidal silver helpful for any condition and is it safe to use?
Find out if there is evidence showing colloidal silver can help to treat infections, boost the immune system, help with arthritis and more, plus find out if colloidal silver can cause blue skin or other side effects.

CL Answer
Energy Gels: What Are They Used For and Do They Contain Their Labeled Ingredients?
Energy gels are a convenient on-the-go carbohydrate source, but some products may not contain the amount of carbohydrates stated on the label.

Recalls & Warnings
March 21, 2024
Eleven Brands of Sexual Enhancement Supplements Recalled Due to Undeclared Prescription Drugs
On March 19, 2024, Pyramid Wholesale issued a recall of 11 brands of sexual enhancement supplements, including honey-based products, after they were found to contain undeclared sildenafil and tadalafil.
CL Answer
What are the health benefits of green tea, and is it safe?
ConsumerLab explains green tea's potential health benefits for everything from fighting cancer to helping your heart.

Recalls & Warnings
July 19, 2023
FDA Warns Seller of Sleepy Time Products and Honey Herbal Syrups for Manufacturing Violations
On June 28, 2023, the FDA issued a warning letter to Eden’s Answers, Inc. after an inspection of the company’s facility found products to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
January 26, 2023
Allergy Bee Nasal Swabs Contaminated With Illness-Causing Bacteria
On January 18, 2023, the FDA issued a warning letter to Buzzagogo, LLC, after the company’s Allergy Bee Gone for Kids nasal swab products were found to be contaminated with bacteria that have the potential to cause life-threatening illness.
CL Answer
Which foods are a good source of B vitamins?
Find out which foods are a good source of B vitamins (like folate and B-12) and when you should take Vitamin B supplements.

CL Answer
Which supplements help with erectile dysfunction?
Supplements for erectile dysfunction. Find out which supplements can help with ED, including ginseng, L-arginine, and maca.

CL Answer
Should you take olive oil as a supplement?
Although extra virgin olive oil has many possible health benefits, such as reduced risk of heart disease and improved blood sugar control, these and other benefits have been demonstrated when olive oil replaces for saturated fats in the diet, not when taken as a supplement, as we explain.

News Release
February 26, 2024
Latest ConsumerLab Survey Shows Growth in Popularity of Magnesium and Several Smaller Supplements
White Plains, New York, February 26, 2024 — A recent survey of more than 10,000 people who regularly use dietary supplements shows that supplements that experienced the greatest absolute growth in popularity during 2023 were pregnenolone (+7.5 percentage points), magnesium (+4.8 pts), berberine (+4.
Recalls & Warnings
June 08, 2022
Allergy Bee Nasal Swabs Recalled Due to Potential Contamination With Yeast, Mold & Bacteria
On June 7, 2022, Buzzagogo, Inc. issued a nationwide recall of one lot of Allergy Bee Gone for Kids Nasal Swab Remedy after FDA testing revealed elevated levels of yeast and mold. The agency also warned the recalled swabs may contain the bacteria Bacillus cereus.
Recalls & Warnings
July 13, 2022
FDA Warns Sellers of Tainted Honey-Based Sexual Enhancement Products
On July 12, 2022, the FDA issued warning letters to four companies selling honey-based products promoted for sexual enhancement after tests conducted by the FDA found the products to contain the prescription drugs Tadalafil and Sildenafil.
Recalls & Warnings
July 15, 2022
Honey-Based Enhancement Supplement Recalled
On July 13, 2022, Shopaax.com issued a recall of all lots of its honey-based sexual enhancement product, Kingdom Honey Royal Honey VIP, after FDA analysis found the presence of undeclared sildenafil.
Recalls & Warnings
December 02, 2020
Seller of Vitamin C, Elderberry, Silver, and More Warned for COVID-19 Claims
On November 10, 2020, the FDA issued a warning letter to Sage Woman Herbs, Ltd.
News Release
February 15, 2024
Unveiling the Truth About Green Tea’s EGCG Content
White Plains, NY, February 15, 2024 — ConsumerLab.com, a trusted name in health and wellness, sheds light on the EGCG (epigallocatechin gallate) content in green tea.
Recalls & Warnings
November 17, 2022
FDA Warns Consumers Not to Use Male Enhancement Supplement
On November 10, 2022, the FDA warned consumers not to buy or use Sangter Natural Male Energy Supplement after FDA laboratory analysis confirmed the presence of undeclared sildenafil.
Recalls & Warnings
January 11, 2023
Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 9, 2022, the FDA issued a warning letter to Distributor RFR, LLC after laboratory analysis of the company’s SANGTER Natural Male Energy Supplement found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
January 19, 2023
Male Sexual Enhancement Supplement Adam’s Secret Found to Contain Prescription Medication
On January 10, 2023, the FDA issued a warning letter to HIS Enterprise Inc dba Adam’s Secret USA, LLC after laboratory analysis found Adam’s Secret Extra Strength 3000 Platinum, Adam’s Secret Extra Strength Blue, Adam’s Secret Extra Strength Purple, Adam's Secret ...
Recalls & Warnings
May 04, 2023
PrimeZEN Enhancement Supplement for Men Found to Contain Prescription Drug
On April 27, 2023, the FDA issued a warning letter to Tager Online, Inc. DBA Volt Candy; Volt Candy Wholesale Club after FDA laboratory analysis determined PrimeZEN Black 6000, a product promoted to improve sexual function for men, contains tadalafil and sildenafil.
Recalls & Warnings
September 01, 2022
FDA Warns Elderberry Fair & Co for Promoting Elderberry Supplements and Apple Cider to Treat Colds & Flu
On August 15, 2022, the FDA issued a warning letter to The Elderberry Fairy & Co., LLC after review of the company’s website found statements about the company’s Elderberry Syrup with Honey, Elderberry Syrup with Agave, and Organic Fire Cider to be drug claims.
Recalls & Warnings
August 03, 2022
Sexual Enhancement Supplement Recalled Due to Sildenafil
On August 1, 2022, DISTRIBUTOR RFR, LLC recalled one lot of SANGTER Energy Supplement 3000 mg to the consumer level after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
October 30, 2020
Seller of Elderberry and Honey Products Warned for COVID-19 Claims
On October 23, 2020, the FDA issued a warning letter to Beepothecary LLC for selling the products BEEHive Delight, BEEbread, and Elderberry, Honey & Propolis Syrup with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 01, 2022
53 Protein, Meal Replacement and Nutritional Beverages Recalled Due to Bacterial Contamination
On July 29, 2022, Lyons Magnus LLC issued a recall of 53 protein, meal replacement, and nutritional beverages, including those sold under the brand names of Glucerna, Premier Protein, Oatly, and others, due to the potential for microbial contamination, including from Cronobacter sakazakii.
Recalls & Warnings
January 03, 2024
FDA Warns Amazon for Selling Men’s Supplements Containing Prescription Drugs
On December 20, 2023, the FDA issued a Warning Letter to Amazon.com, Inc.
News Release
October 21, 2020
Best Apple Cider Vinegar? Quality of Bottled Apple Cider Vinegars and Supplements Varies, According to ConsumerLab
White Plains, New York, October 21, 2020 — Apple cider vinegar is often promoted for lowering blood sugar levels, weight loss, "balancing" pH, and improving digestion.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Turmeric, Ashwagandha, Glucosamine, and More
On December 18, 2020, the FDA issued a warning letter to Reddy Naturals following a review of the company's website, which found statements made about the company's products Glycostasis, Glucosamine Curcumin, Turmeric 50, Turmeric Curcumin, Cetyl Curcumin, Ashwagandha, Huruli, ...
Recalls & Warnings
September 29, 2022
Sexual Enhancement Supplement Sold on Amazon and Walmart Recalled Due to Undeclared Drugs
On September 27, 2022, Proper Trade LLC/My Stellar Lifestyle recalled two lots of Wonder Pill after Amazon laboratory analysis found the product to contain undeclared tadalafil, a prescription medication.
Recalls & Warnings
February 13, 2023
FDA Finds Prescription Drug in “100% Natural” Weight Loss Supplement
On February 8, 2023, the FDA warned consumers not to buy or use Alfia 100% Natural Weight Loss Capsules after FDA laboratory analysis confirmed the presence of sibutramine.
Recalls & Warnings
July 26, 2023
Gadget Island, Inc. Male Enhancement Supplements Found to Contain Prescription Drugs
On July 21, 2023, the FDA issued a warning letter to Gadget Island, Inc.
News Release
May 10, 2018
Big Differences Found Among Green Teas -- ConsumerLab Tests Identify Best Green Tea in Bags, Matcha, Supplements, and Bottles --
White Plains, New York, May 10, 2018 — The benefits of green tea use are associated with the presence of natural polyphenols known as catechins, such as EGCG.
News Release
October 19, 2017
ConsumerLab.com Reveals Best Zinc Lozenges and Supplements for Shortening Colds and Correcting Deficiency
White Plains, New York, October 19, 2017 — In the U.S., mild zinc deficiency is fairly common. Zinc supplements can help to correct a deficiency, slow the progression of age-related macular degeneration, and have other benefits. Taken as a lozenge, zinc can shorten the duration of a cold.
News Release
February 19, 2015
Only 50% of Aloe Products Pass ConsumerLab.com Tests
White Plains, New York, February 19, 2015 — "Choose aloe products carefully," cautions ConsumerLab.com president Tod Cooperman, M.D. after recent tests found only five of 10 aloe pills, gels and drinks selected for review to contain what the company expected based on labels.
News Release
December 21, 2012
ConsumerLab.com Tests Reveal What's Really In Green Tea Supplements and Bottled Drinks
White Plains, New York — December 21, 2012 — How do levels of EGCG and caffeine in green tea supplements and bottled teas compare with levels provided in a typical brewed green tea? It very much depends on the brand, according to new tests by ConsumerLab.com.
Recalls & Warnings
May 12, 2020
FDA Warns Seller of "COVID-19 Cough Syrup"
On May 8, 2020, the FDA issued a warning letter to Seanjari Preeti Womb Healing, L.L.C. for selling its product COVID-19 Cough Syrup with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
July 10, 2020
Sundial Herbal Recalls More Than Sixty Products Including Turmeric, Cinnamon & Tea
On July 9, 2020, Sundial Herbal Products issued a recall of more than 60 herbal products because they were misbranded and/ or promoted with drug claims. Their labels contain drug claims stating the products can diagnose, cure, mitigate, treat, or prevent disease.
Recalls & Warnings
July 26, 2022
Sexual Enhancement Supplement Sold on Amazon Recalled
On July 21, 2022, Ultra Supplements LLC issued a recall of one lot of Sustango capsules after Amazon laboratory analysis found the presence of the prescription drug Tadalafil. The company has received no reports of adverse effects related to this recall to date.
Recalls & Warnings
March 26, 2019
LEOPARD Miracle Honey Recalled
On March 22, 2019, USA LESS issued a recall of all lots of LEOPARD Miracle Honey, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
June 20, 2020
FTC Warns 30 More Companies for Coronavirus Claims
On June 18, 2020, the FTC announced that it sent warning letters to 30 companies for selling products such as immune system boosters, colloidal silver, vitamin C, and CBD with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Flu Immune
On December 21, 2020, the FDA issued a warning letter to Riverstone LLC for selling the products Flu Immune Drops, L-Lysine, Lysine Extra, and Monolaurin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Liposomal Vitamin C, Vitamin D & More
On December 21, 2020, the FDA issued a warning letter to Sparrow Health & Performance LLC for selling the products Organic Liposomal Vitamin C, Nanoemulsified D3K2 (also called Liquid Liposomal Vitamin D3 with K2 or Liposomal Vitamin D3) and Immune Support ...
Recalls & Warnings
November 02, 2020
Silver Cannot Be Promoted to Prevent or Treat COVID-19
On October 30, 2020, the FDA issued a warning letter to Spartan Enterprises Inc. dba Watershed Wellness Center for selling the silver product Dissolve BioActive Silicate with unsupported claims that it can treat coronavirus (COVID-19).
Recalls & Warnings
December 11, 2020
Colloidal Silver Seller Warned for COVID-19 Claims
On December 2, 2020, the FDA issued a warning letter to Heavenly Natural Products for selling various Carbon 60 and colloidal silver products, including AVOCADO C60 ANTI-VIRAL COMBO — VIRUS PREVENTION (made with C60 WITH AVOCADO and Heavenly Silver) with unsupported ...
Recalls & Warnings
December 11, 2020
Seller of "Dr. Hotze's Immune Pak" Products Warned for COVID-19 Claims
Seller of "Dr. Hotze's Immune Pak" Products Warned for Coronavirus Claims
Recalls & Warnings
December 11, 2020
FDA Warns iThrive.health for Promoting Supplements as COVID-19 Treatments
On December 10, 2020, the FDA issued a warning letter to iThrive.health for promoting the sale of various vitamins and supplements on Amazon.com with claims that they can mitigate, prevent, treat, diagnose, or cure COVID-19.
Recalls & Warnings
April 10, 2021
HI-TECH Workout Supplements Recalled Due to Undeclared Allergens
On April 3, 2021, HI-TECH Pharmaceuticals issued a recall of all lots of APS Nutrition Isomorph 28 in a 2lb jug because they contain undeclared milk, wheat, and soy.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
December 01, 2020
Niagen Cannot Be Promoted to Prevent or Treat COVID-19, Warns FDA
On November 17, 2020, the FDA issued warning letters to the sellers of Niagen and Nadovim, supplements that contain different forms of niacin, for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 12, 2014
Seller of Omega-3 "Syrup" Warned for Manufacturing Violations and Drug Claims
On October 16, 2014, the FDA issued a warning letter to Mr.
Recalls & Warnings
July 18, 2017
Hidden Drugs Found in Sexual Enhancement Products
On July 17, 2017, the FDA posted public notifications after finding multiple sexual enhancement products contained hidden drug ingredients.
Recalls & Warnings
October 26, 2019
Green Lumber Enhancement Supplements Recalled
On October 22, 2019, GL Holdings issued a recall of Green Lumber, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain tadalafil.
Recalls & Warnings
September 21, 2019
Liquid Vitamin C for Men Recalled
On September 16, 2019, Fitoterapia USA Inc. issued a recall of Macho Artificial Passion Fruit Flavored Vitamin C Liquid Supplement, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain tadalafil.
Recalls & Warnings
December 01, 2018
Sexual Enhancement Supplements Contain Prescription Drugs
The FDA recently warned consumers that the following sexual enhancement supplements were found to contain undeclared sildenafil and/or tadalafil. Use the links below to read the complete warning letter for each:
- Willy Go Wild
Recalls & Warnings
July 18, 2017
FDA Warns Seller of "Quick Slim with pure Hoodia Gardonii" and "Diabalance Herbal Blood Sugar Balance"
On July 11, 2017, the FDA issued a warning letter to Black Seed Herb, Inc.
Recalls & Warnings
December 19, 2020
FDA Finds Unapproved Drugs in Many Weight Loss and Sexual Enhancement Products Sold Online
On December 18, 2020, the FDA warned consumers that certain products promoted for weight loss, body building, sexual enhancement, pain relief, sleep, and other uses because may contain harmful ingredients.
Recalls & Warnings
June 23, 2016
Protein Drinks Recalled Due to Possible Spoilage
On June 22, 2016 Bolthouse Farms issued a recall of the protein drinks listed below due to possible spoilage that may cause the beverages to appear lumpy, taste unpleasant and have an off odor.
Recalls & Warnings
March 06, 2015
Seller of Tea Drinks Warned for Drug Claims
On February 26, 2015, the FDA issued a warning letter to the seller of Anamu CancerHerb Tea Box, Anamu CancerHerb Tea Box with Honey and Organic Bottled Tea Drinks because statements made about these products on the websites, www.cancerherbtea.com and www.anamucancerherbtea.
Recalls & Warnings
July 11, 2014
Maker of Cough and Sleep "Remedies" Warned for Drug Claims
On June 27, 2014, the FDA issued a warning letter to Zarbee's, Inc.
Recalls & Warnings
December 18, 2018
Metal Flakes Reported in Zarbee's Naturals Gummy Multivitamin
Metal flakes were found in a bottle of Zarbee's Naturals Complete Toddler Multivitamin gummies and at least one other Zarbee's product, according to reports by Fox 13 News (Salt Lake City) and USA Today.
Recalls & Warnings
May 15, 2019
Titanium 4000 Capsules for Men Recalled
On May 7, 2019, D.B.P. Distribution issued a recall of Titanium 4000, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil and tadalafil.
Recalls & Warnings
May 07, 2019
"The Beast" Supplement for Men Recalled
On May 7, 2019, STIFF BOY LLC issued a recall of The Beast, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
April 12, 2019
Sexual Enhancement Supplement for Men Recalled
On April 9, 2019, SD Import, LLC issued a recall of Aphrodisiac, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
April 08, 2019
Herbal "Coffee" for Sexual Enhancement Recalled
On March 22, 2019, Brian Richardson DBA "In Tha Pink" issued a recall of ground Kopi Jantan Tradisional [sic] Natural Herbs Coffee, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil and tadalafil.
Recalls & Warnings
December 05, 2014
Maker of Immune Supplement Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Urban Moonshine, Inc.
Recalls & Warnings
February 10, 2015
Seller of "Natural" Cough Syrup Warned for Manufacturing Violations, Drug Claims
On January 21, 2015, the FDA issued a warning letter to Fragrance Manufacturing Incorporated, following a facility inspection which found the company's products, including Maty's All Natural Cough Syrup and Maty's All Natural Cough Syrup for Kids, to be adulterated because they ...
Recalls & Warnings
November 06, 2014
Seller of Cholesterol, Arthritis Supplements and More Warned for Drug Claims
On October 17, 2014, the FDA issued a warning letter to Vitamins Direct (USA) and Golden Pride, Inc., which distribute Flexezy and Physician's Signature brands of dietary supplements, following a facility inspection which found statements made about certain supplements to be drug claims.






