Reviews and Information for NAD Boosters (NAD+/NADH, Nicotinamide Riboside, and NMN)
NAD Boosters (NAD+/NADH, Nicotinamide Riboside, and NMN)
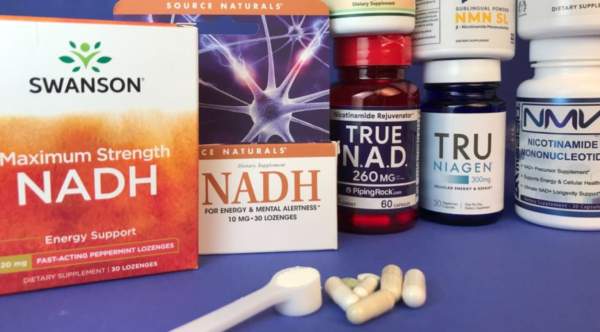
Several supplements can boost levels of NAD in the body, but does it matter and how do these products compare? Find out in this review in which ConsumerLab purchased and tested the quality of popular NAD+, NADH, nicotinamide riboside, and NMN supplements and evaluated the research behind them.
NAD Boosters (NAD+/NADH, Nicotinamide Riboside, and NMN) ReviewSearch term may appear only in full report available to members. Join now for full access.
CL Answer
Is it okay to take vitamin D3 and fish oil together?
Find out if taking vitamin D and fish oil is okay, how it effects absorption, and the best way to take vitamin D to improve absorption. ConsumerLab.com's answer explains.

News Release
November 05, 2021
NAD Boosters for Energy and Memory? ConsumerLab Reviews NAD+, NAHD and NMN Supplements
White Plains, New York, November 5, 2021 — Most cells in the body use NAD (nicotinamide adenine dinucleotide) in the process of energy production, and compounds that boost blood levels of NAD are often sold in supplements marketed for a wide range of benefits, from increasing energy and ...
Recalls & Warnings
December 01, 2020
Niagen Cannot Be Promoted to Prevent or Treat COVID-19, Warns FDA
On November 17, 2020, the FDA issued warning letters to the sellers of Niagen and Nadovim, supplements that contain different forms of niacin, for selling products with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
May 09, 2020
FDA Warns Sellers of Essential Oils, CBD, Vitamins, and More Promoted to Treat Coronavirus
Between May 7 and May 8, 2020, the FDA issued warning letters to seven companies for selling products such as essential oils, CBD, hand sanitizers, and vitamins with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
December 06, 2013
Weight Loss Supplement Claims Challenged
On November 27, 2013, the National Advertising Division (NAD) recommended HealthyLife Sciences, LLC, modify or discontinue the use of certain claims made about the company's weight loss supplement Healthe Trim.
Recalls & Warnings
April 12, 2013
Zicam Advised Against Using Cold Prevention Claim In Advertising
On April 5, 2013, it was reported that the National Advertising Division (NAD) recommended that Matrixx Initiatives, maker of Zicam cold products, stop making claims in its advertising that the product can prevent colds.
Recalls & Warnings
September 13, 2014
Marketer Banned From Selling Weight Loss Products
In order to settle FTC charges of deceptive weight loss claims, John Matthew Dwyer III, the co-founder of HealthyLife Sciences, LLC, has agreed to no longer manufacture or market weight loss supplements.