Reviews and Information for Ensure
Search term may appear only in full report available to members. Join now for full access.
Product Review
Cinnamon Supplements and Spices Review
CAUTION: Some Cinnamon Products High in Toxin. See Which Passed or Failed and our Top Picks!

Product Review
Water Filter Pitchers Review
See the Best Pitchers For Different Filtering Needs

Product Review
Whole, Ground, Milled, and Cracker Flaxseed Review
High Levels of Cadmium Found in Flaxseed Products — Testing Expanded

CL Answer
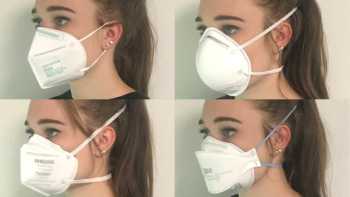
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

Product Review
Red Yeast Rice Supplements Review
50% of Red Yeast Rice Supplements "NOT APPROVED" in CL Tests

Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Our Top Picks and Which 5 Failed Testing

Product Review
Probiotic Supplements Review (Including Pet Probiotics)
Probiotics: See What They Really Contain and Our Top Picks

Product Review
Canned Tuna, Salmon, Sardines & Mackerel Review
Find Fish With the Least Mercury & Arsenic and Most Omega-3s

CL Answer
7 Tips to Avoid Getting a Pill Stuck in Your Throat
Some pills can be difficult to swallow and can even become lodged in the throat or cause damage to the esophagus. Learn seven practical tips to help avoid getting pills stuck in the throat.

CL Answer
Supplements That May Be Needed When Following a Gluten-Free Diet
Find out if supplements such as folate, vitamin B-12, niacin, thiamin, iron, vitamin D, and/or fiber are needed when following a gluten-free diet. Also learn how to find gluten-free supplements on ConsumerLab.com.

Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

Product Review
Garcinia Cambogia (HCA) Supplements Review
Find the Best Garcinia Cambogia Supplement. Choose Carefully! Most Garcinia (HCA) Weight Loss Supplements Lack Listed Amount of Ingredient .

Product Review
Gluten-Free Grains Review (Buckwheat, Quinoa, Sorghum, Millet, and Teff)
31% of Gluten-Free Grains Fail Our Tests Due to Contaminants. Quinoa, Buckwheat, Sorghum, Millet and Teff Tested.

Product Review
Psyllium Fiber Supplements Review
Find the Best Psyllium Fiber Supplement and Avoid Lead.

Product Review
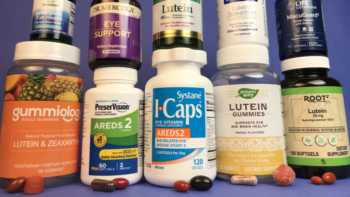
Vision Supplements Review (with Lutein, Zeaxanthin & AREDS2 Formulas)
Find the Best Vision Supplement Based Our Tests
Product Review
Quercetin & Rutin Supplements Review
Quality's a Concern With Quercetin and Rutin Supplements -- Only 17% of Claimed Amount In One

CL Answer
What is the best sunscreen based on safety and efficacy?
We’ve boiled down the latest research about mineral and chemical sunscreens, including product tests, to help you choose the best sunscreen (which will depend on whether you expect to be in or out of the water). We also list popular sunscreens that have, or have not, been found to contain cancer-causing compounds (benzene and benzophenone).

CL Answer
Energy Gels: What Are They Used For and Do They Contain Their Labeled Ingredients?
Energy gels are a convenient on-the-go carbohydrate source, but some products may not contain the amount of carbohydrates stated on the label.

Clinical Update
9/19/2023
Protein After Hospitalization
Does a protein drink like Ensure Protein help with recovery after hospitalization? See what a study showed in the Recovery section of our Protein Review, which includes our Top Picks among protein powders and drinks.
Clinical Update
4/09/2024
Magnesium Glycinate – What to Look For
Some magnesium glycinate supplements are not truly this chelated form of magnesium. Find out what to look for on labels to help ensure you’re getting chelated magnesium glycinate in the ConsumerTips section of our Magnesium Supplements Review, which includes our Top Picks for magnesium.
Clinical Update
9/28/2022
Plant Protein Pairings
What are the best combinations of plant-based foods to ensure proper intake of essential amino acids? Find out in the ConsumerTips section of our Protein Supplements Review.
Product Review
Muscle & Workout Supplements Review (Creatine and Branched-chain Amino Acids)
Do Creatine and BCAAs Really Improve Strength and Recovery?

CL Answer
What’s the Best Way to Get High-Quality Protein from Food?
Find out the best way to get high-quality protein from animal or plant-based food instead of supplements.

CL Answer
Can I trust ConsumerLab.com? How are its tests paid for?
Learn more about ConsumerLab.com's clinical testing policy and procedures, including information on our independent testing and product reviews. Our answer explains.

CL Answer
I've tried a number of fish oil supplements, all of which make me nauseous. What is the best non-fish alternative? I am primarily interested in getting EPA.
Learn how to reduce fish oil side effects such as nausea and upset stomach.

CL Answer
Are gummy vitamins better or worse than pills?
Learn more about gummy supplements, including those for multivitamins, vitamins C and D, calcium, and B vitamins.

Product Review
Baobab Dried Fruit Pulp Review Article
What Are the Benefits of Baobab Dried Fruit Pulp? Find Out What's In Baobab Super Fruit Products, Safety, Side Effects & More.

CL Answer
10 Supplements That May Help Reduce Nighttime Urination (And 5 That May Not)
Waking up more than once during the night to use the bathroom? Learn more about the 9 supplements that may help and 5 others that don't.

CL Answer
Are Balance of Nature products good dietary supplements?
Do Balance of Nature supplements "Fruits" and "Veggies" supplements really provide the daily recommended servings of fruits and vegetables, and are they worth the cost? Find out.

CL Answer
Is Ester-C the best form of vitamin C?
Info on Ester-C Vitamin C, including which form of vitamin C is best, fully-reduced, liposomal and others based on absorption and side effects. ConsumerLab.com's answer explains.

Recalls & Warnings
November 09, 2023
InnoMark Warned for Manufacturing & Misbranding Violations
On September 1, 2023, the FDA issued a Warning Letter to InnoMark, Inc.
Recalls & Warnings
June 19, 2024
DBH Beverly Hills Sunscreen Falsely Promoted as Octinoxate Free, Says FDA
On June 12, 2024, the FDA issued a Warning Letter to Aqualex Co., Ltd.following inspection of the company’s website, manufacturing facility, and sunscreen products, which found its DBH Beverly Hills, EGF FGE DNA, UV Shield sunscreen to be misbranded.
CL Answer
Do any supplements or lifestyle changes reduce the symptoms of tinnitus? Is it true that some supplements can cause tinnitus?
Learn which supplements can ease tinnitus, including melatonin and pine bark extract. Understand which may actually cause tinnitus.

CL Answer
Where to Safely Buy Real Vitamins and Supplements Online, Not Fakes or Counterfeits
ConsumerLab explains how to avoid counterfeit vitamins and supplements when shopping online on Amazon, Walmart.com, and other sites. Learn to identify authorized sites and sellers and avoid fake supplements. Use the brand-by-brand guide to protect yourself from risk.

Recalls & Warnings
August 16, 2023
FDA Warns Sun Ten Laboratories for Manufacturing Violations, Drug Claims
On April 7, 2023, the FDA issued a warning letter to STPCA Inc. dba Sun Ten Laboratories because products were found to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
February 22, 2024
Pacific BioLogic Co. Warned by FDA for Manufacturing Violations
On December 21, 2023, the FDA issued a Warning Letter to Curtis Jacquot, dba Pacific BioLogic Co.
CL Answer
Can drinking coffee prevent gallstones? Can supplements help?
Information about the impact coffee has on gallstone disease. Plus, supplements that have been proposed to help with gallstone pain.

Recalls & Warnings
September 03, 2019
Federal Court Shuts Down Two Supplement Companies Selling Weight, Joint Health and Other Supplements
On September 3, 2019, a U.S. District Court entered a consent decree of permanent injunction against Basic Reset and Biogenyx, two Tennessee-based companies that sell dietary supplements and other product promoted for health benefits.
Recalls & Warnings
July 17, 2023
Seller of Ashwagandha, Lion’s Mane & Other Supplements Warned for Manufacturing Violations
On March 17, 2023, the FDA issued a warning letter to Brand Packaging Group, Inc. after FDA inspection of the company’s facility found products to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
July 19, 2023
FDA Warns Seller of Sleepy Time Products and Honey Herbal Syrups for Manufacturing Violations
On June 28, 2023, the FDA issued a warning letter to Eden’s Answers, Inc. after an inspection of the company’s facility found products to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
December 26, 2023
Total Body Nutrition, TBN Labs, and Loud Muscle Science Agree to Stop Selling Adulterated and Misbranded Dietary Supplements
On December 13, 2023, Total Body Nutrition LLC, TBN Labs LLC, and Loud Muscle Science LLC, as well as the companies’ owner, Mohammed Islam, agreed to stop manufacturing and distributing adulterated and misbranded dietary supplements and to comply with dietary supplement current good ...
News Release
January 09, 2025
Not All Black Seed Oil Supplements Contain What They Claim, According to ConsumerLab
White Plains, NY, January 9, 2025 — Black seed oil and extract supplements are promoted for controlling blood pressure, cholesterol, blood sugar, and arthritis pain, and more.
News Release
December 20, 2024
Best Zinc Supplements and Lozenges for Colds, According to ConsumerLab
White Plains, NY, December 20, 2024 — Zinc is important for a healthy immune system and the right zinc lozenge can help reduce cold symptoms, but taking too much zinc causes adverse effects.
News Release
October 31, 2024
Best SAMe Supplements for Arthritis and Depression, According to ConsumerLab Tests
White Plains, NY, October 31, 2024— SAMe (S-adenosyl-methionine) supplements may reduce joint pain, stiffness, and inflammation caused by osteoarthritis, as well as improve symptoms of depression.
Recalls & Warnings
April 24, 2023
FTC Warns Almost 700 Companies: Unsupported Health Claims May Result in Substantial Civil Penalties
On April 13, 2023, the Federal Trade Commission (FTC) notified approximately 670 companies that market over-the-counter (OTC) drugs, homeopathic products, dietary supplements, or functional foods that they could incur significant civil penalties if they make unsubstantiated claims about the ...
Recalls & Warnings
December 29, 2022
EarthLab, Inc. Warned for Promoting Curcumin, Elderberry to Treat Pain & Flu
On November 10, 2022, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals following inspection of the company’s website which found statements about the company’s products to be drug claims because they suggest the products can prevent or treat disease.
Recalls & Warnings
March 03, 2012
FDA Warns Vitaganic of Manufacturing Violations
On February 8, 2012, the U.S. FDA sent a Warning Letter to Vitaganic, Inc. regarding violations of Current Good Manufacturing Practic (CGMP) regulations for dietary supplements found during an inspection of it manufacturing facility in Sunnyvale, California in October 2011.
Recalls & Warnings
August 14, 2020
The Green Herb and New Genesis Health Warned for Manufacturing Violations
On July 31, 2019, the FDA issued a warning letter to Davis Ventures, Inc.
Recalls & Warnings
June 30, 2022
FDA Warns Seller of Vision and Allergy Supplements
On May 26, 2022, the FDA issued a warning letter to Golden Lab LLC following an inspection of the website, which found statements about the company’s DoctoRx’s Optimal Formula Ocular Pressure & Optic Nerve Support Formula Ocular Health Capsule, DoctoRx’s Optimal Formula ...
Recalls & Warnings
January 03, 2022
Seller of Wormwood, Aloe Supplement Warned by FDA
On December 7, 2021, the FDA issued a warning letter for Western Herb Products, Inc.
News Release
July 31, 2024
Best Calcium & Bone Health Supplements According to ConsumerLab Tests
White Plains, NY, July 31, 2024 — Calcium is essential to maintaining bone health and plays critical roles in nerve transmission, muscle contraction, and the cardiovascular system.
News Release
August 24, 2018
Few Red Yeast Rice Supplements Provide Enough Cholesterol-Lowering Compounds to Likely Be Effective, ConsumerLab Tests Reveal
White Plains, New York, August 24, 2018 — Research shows that red yeast rice, which contains naturally-occurring lovastatin compounds, can lower "bad" LDL cholesterol.
News Release
July 19, 2017
New Tests Reveal Whether Dark Chocolates and Cocoas Are Toxic or Healthful
White Plains, New York, July 19, 2017 — Dark chocolates and cocoa may contain dangerously high levels of the heavy metal cadmium, a kidney toxin, according to recent tests conducted by ConsumerLab.com, an independent evaluator of health and nutrition products.
News Release
April 25, 2017
Caution with Kelp Supplements: May Contain Too Much Iodine
White Plains, New York, April 25, 2017 — Kelp supplements are promoted as a natural source of iodine, an important mineral for healthy thyroid function. But are they a good alternative to regular iodine supplements? To find out, ConsumerLab.
News Release
December 06, 2013
Retesting Confirms Lack of Ingredient in Garcinia Cambogia Supplement
White Plains, New York, December 6, 2013 — Retesting of a dietary supplement reported in October to contain significantly less of a key ingredient than listed on its label corroborates the original findings from ConsumerLab.com.
News Release
May 23, 2011
Red yeast rice supplements weaker now than in 2008; Wide variation among brands and contamination discovered by ConsumerLab.com -- Popular cholesterol-lowering supplements tested and compared
WHITE PLAINS, NEW YORK — MAY 23, 2011 — ConsumerLab.com announced today that tests of eleven red yeast rice supplements revealed enormous differences in levels of cholesterol-lowering statin compounds. Statin levels fell dramatically among brands previously tested in 2008.
News Release
August 20, 2007
ConsumerLab tests products in booming nutrition drink market — most deliver what's promised but extra cholesterol found in some — New report covers drinks and powders for endurance/recovery, body building, meal-replacement and dieting
WHITE PLAINS, NEW YORK — AUGUST 20, 2007 — A new report from ConsumerLab.com finds most nutrition powders, shakes and drinks meet their nutrient claims, but three products — two sports drinks and a meal supplement — contained more cholesterol than claimed.
News Release
July 17, 2007
Concerned by weaknesses in FDA's new rules for dietary supplements, ConsumerLab.com acts to "raise the bar on quality"
WHITE PLAINS, NEW YORK — July 17, 2007 — Disappointed by the lack of quality standards within the FDA's newly released Good Manufacturing Practices (GMPs) for dietary supplements, ConsumerLab.com has announced two initiatives today intended to "raise the bar on quality.
News Release
December 12, 2006
Probiotic supplements grow in popularity but viable bacteria missing in many — ConsumerLab.com cautions consumers to select probiotics carefully and store them properly
WHITE PLAINS, NEW YORK — DECEMBER 12, 2006 — Sales of supplements containing beneficial organisms (probiotics) have grown rapidly, but a new test report from ConsumerLab.com shows that 44% contained fewer viable organisms than claimed or generally known to be effective.
News Release
August 25, 2004
ConsumerLab.com finds not all vitamin E products meet claims — Results for 33 vitamin E supplements and skin products released today
WHITE PLAINS, NY — Wednesday, August 25, 2004 (Updated 10/24/04) — ConsumerLab.com announced today that five vitamin E products failed to pass recent testing for having either too little vitamin E and/or for containing synthetic vitamin E when claiming to be natural.
News Release
March 27, 2001
ConsumerLab.com expands testing of health products; success of dietary supplement evaluations leads company into broader nutrition field, beginning with popular nutrition bars
WHITE PLAINS, NY — March 27, 2001 — Having established its presence as the leading independent evaluator of the quality of dietary supplements sold in the U.S, ConsumerLab.com announced today the expansion of its work to include foods and food ingredients.
News Release
March 13, 2001
ConsumerLab.com review of vitamin E supplements finds some insufficiencies and need for clearer labeling; test results released online today
WHITE PLAINS, NY — March 13, 2001 — ConsumerLab.com today released results of its Product Review of vitamin E products. Vitamin E is one of the most popular dietary supplements in the U.S.
News Release
February 14, 2001
Problems found with multivitamins and multiminerals by ConsumerLab.com
WHITE PLAINS, NY — February 14, 2001 — ConsumerLab.com announced today that only 14 of 27 products evaluated in its Multivitamin and Multimineral Product Review achieved full "CL Approved Quality" status.
News Release
January 29, 2001
Some supplements for arthritis may exceed newly released safe intake levels for manganese
WHITE PLAINS, NY — January 29, 2001 — ConsumerLab.com issued an alert today to its online readers that that some glucosamine and chondroitin products (popularly used for osteoarthritis), exceed newly established upper intake levels for manganese.
News Release
November 21, 2000
ConsumerLab.com tests popular supplement for heart failure; CoQ10 test results released online today
WHITE PLAINS, NY — November 21, 2000 — ConsumerLab.com today released results of its 10th Product Review, focusing on dietary supplements containing coenzyme Q10 (CoQ10).
News Release
September 06, 2000
ConsumerLab.com finds new calcium products of higher quality than many traditional supplements; Test results released online today
WHITE PLAINS, NY, September 6, 2000 — ConsumerLab.com today released results of its 9th Product Review, focusing on dietary supplements and products providing calcium. One of the most commonly purchased supplements in the U.S.
News Release
August 07, 2000
ConsumerLab.com finds that not all creatine supplements meet label claims; Popular sports supplement test results released online
WHITE PLAINS, NY, August 7, 2000 — ConsumerLab.com today released results of its 8th Product Review, focusing on creatine monohydrate dietary supplements.
News Release
July 11, 2000
Pesticide contamination found in many Ginseng supplements tested by ConsumerLab.com
WHITE PLAINS, NY, July 11, 2000 — Out of 22 brands of ginseng dietary supplements evaluated by ConsumerLab.com eight were found to contain high levels of specific pesticides, some of which also contained significant levels of lead.
Recalls & Warnings
April 27, 2023
Seller of Magtein, Veggie Caps, & More Warned for Drug Claims, Manufacturing Violations
On March 8, 2023, the FDA issued a warning letter to Spartan Enterprises Inc., dba Watershed Wellness Center, after a review of the company’s website found statements about its Dr. Bob’s Naturals Spirulina, Dr. Bob’s Naturals Magtein, Dr.
News Release
May 17, 2000
ConsumerLab.com test of vitamin C products identifies quality control issues and misuse of USP claim.
WHITE PLAINS, NY, May 17, 2000 — Testing of 26 brands of vitamin C dietary supplements indicated that fifteen percent either did not contain all of the claimed ingredient or failed to breakdown as needed for absorption in the body.
News Release
March 28, 2000
Only half of SAM-e supplements found to contain proper ingredients and labeling in ConsumerLab.com testing; Product Review of popular anti-depressant published online today.
WHITE PLAINS, NY, March 28, 2000 — Among 13 brands of SAM-e dietary supplements (used primarily in treating depression and osteoarthritis) only seven were found to contain labeled amounts of SAM-e (S-adenosyl-methionine). These results were reported today by ConsumerLab.
News Release
March 07, 2000
ConsumerLab.com finds many arthritis supplements lacking labeled ingredients; Glucosamine and Chondroitin product review published online today.
WHITE PLAINS, NY, March 7, 2000 — Among 25 major brands of supplements containing glucosamine and/or chondroitin used for treating osteoarthritis, nearly one-third were found not to contain all labeled ingredients. These results were reported today by ConsumerLab.
News Release
January 31, 2000
Quality of prostate supplements get mixed review in ConsumerLab.com study; Saw palmetto product review published online today.
WHITE PLAINS, NY, January 31, 2000 — Out of 27 major brands of dietary supplements purporting to contain saw palmetto for treating symptoms of prostate enlargement, only 17 appeared to have ingredients similar to those found in published clinical studies.
Recalls & Warnings
April 16, 2012
Protein Supplement Maker Fails FDA Audit -- Many Products Affected
The FDA sent a Warning Letter (dated April 2, 2012) to Theta Brothers Sports Nutrition, Inc. regarding numerous violations of good manufacturing practices at its Lakewood, New Jersey facility.
Recalls & Warnings
February 17, 2010
FTC Warns of Deceptive Claims with Children's Omega-3 Supplements
On February 17, 2010, the Federal Trade Commission (FTC) announced that it had sent letters to 11 companies that promote various omega-3 fatty acid supplements, telling them they should review their product packaging and labeling to make sure they do not violate federal law by making baseless ...
Recalls & Warnings
October 30, 2014
Supplement Maker Warned for Manufacturing Violations
On October 21, 2014, the FDA issued a warning letter to DNG Trading & Milling, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
May 08, 2021
FDA Warns Seller of Vitamins B12, C, D, and K
On April 7, 2021, the FDA issued a warning letter to Unived Inc following a review of the company's websites, which found statements made about the company's products Unived brand Colox, CalDveg, B12+D3, and D3+K2-7 to be drug claims.
Recalls & Warnings
September 25, 2018
Seller of B Vitamins, Multis, Glucosamine & More Warned for Manufacturing Violations
On August 31, 2018, the FDA issued a warning letter to Independent Nutrition Inc., following a facility inspection which the company's products, including B-50 Complete, Multi-Vitamin & Mineral Complex (a.k.a.
Recalls & Warnings
June 16, 2020
Seller of Silver, Arginine, and More Warned for Manufacturing Violations
On June 1, 2020, the FDA issued a warning letter to Morningstar Minerals LLC, which found the company's products, including Silver Boost, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
June 05, 2020
Seller of CBD, Sleep Aids, and Cold & Flu Products Warned for Manufacturing Violations
On April 28, 2020, the FDA issued a warning letter to The Dragontree Apothecary LLC, which found the company's Sleep Support, Anxiety Relief, Cold & Flu Relief, and Inflammation Relief to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
June 02, 2020
Sundial Herbal Ordered to Halt Distribution of Products
On June 1, 2020, a New York federal judge filed an order against two individuals doing business as Sundial Herbal Products to stop the company from distributing its products, which were found to be misbranded and promoted as drugs.
Recalls & Warnings
December 27, 2019
Federal Court Shuts Down Three Supplement Companies With Serious Manufacturing Violations
Update: (1/23/20) ABH has issued a recall of all its products, which are sold under various brand names by over 800 distributors and retailers. More details are available in CL's post about this recall.
Recalls & Warnings
November 28, 2014
Seller of Herbal Extracts Warned for Manufacturing Violations, Drug Claims
On November 3, 2014, the FDA issued a warning letter to Avena Botanicals, Inc.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
May 14, 2014
Maker of Mood, Smoking Cessation Supplements and More Warned for Violations, Drug Claims
On April 17, 2014, the FDA issued a warning letter to CDJ Holding, Incorporated, d.b.a.
Recalls & Warnings
July 18, 2018
Maker of Vitamin B12 and Multimineral Supplements Warned by FDA
On July 6, 2018, the FDA issued a warning letter to Aegle Nutrition, LLC, following a facility inspection which found some of the company's products, including Tropical Oasis Ionized Trace Minerals, Tropical Oasis Ultra Methyl B12, and Tropical Oasis Premium Vitamin B12 to be ...
Recalls & Warnings
June 21, 2010
FDA Warns Parents to Be Careful Giving Vitamin D Drops to Infants
On June 15, 2010, the U.S. FDA (Food and Drug Administration) alerted parents and caregivers that some liquid Vitamin D supplement products are sold with droppers that could allow excessive dosing of Vitamin D to infants.
Recalls & Warnings
February 28, 2013
Maker of Liquid Omega-3 Supplements Warned For Manufacturing Violations
On February 20, 2013, the FDA issued a warning letter to Capco Custom Packaging Inc.
Recalls & Warnings
July 15, 2014
Vita Springs Health Warned for Manufacturing Violations and Drug Claims
On June 24, 2014, the FDA issued a warning letter to Albert Max Inc.
Recalls & Warnings
November 30, 2011
Maker of Acai Product Warned by FDA of Manufacturing Violations
The U.S. FDA recently published a Warning Letter to Precision Formulations, LP (Coppell, Texas) dated 10/24/11, indicating that a recent inspection of its facility found violations of the Current Good Manufacturing Practice reguations for dietary supplements.
Recalls & Warnings
April 03, 2014
Maker of Green Coffee Bean Extract and Weight Loss Supplement Warned for Manufacturing Violations
On March 13, 2014, the FDA issued a warning letter to Libi Labs, Inc.
Recalls & Warnings
November 27, 2011
FDA Seeks Permanent Injunction Against Dietary Supplement Maker -- 400+ Products Affected
On November 23, 2011, the U.S. FDA took legal action against a dietary supplement maker and owner for substituting ingredients and products without noting the changes on the final product labels. The permanent injunction, filed on behalf of the FDA by the U.S.
Recalls & Warnings
June 21, 2011
FDA Warns Laboratory of Manufacturing Violations
On June 10, 2011, the U.S. FDA posted a Warning Letter (dated 4/15/11) to Nutro Laboratories, a division of NBTY, Inc., concerning serious violations of the current Good Manufacturing Practice (CGMP) regulations for Dietary Supplements based on its inspection of Nutro facilities in October 2010.
Recalls & Warnings
March 28, 2011
FDA Cracks Down on Violators of Supplement Manufacturing Rules
In February and March 2011, the U.S. FDA posted Warning Letters sent, respectively, to a distributor of dietary supplements and a supplement manufacturer for violations of current Good Manufacturing (GMP) rules.
Recalls & Warnings
August 06, 2014
Seller of Mineral and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to Mezotrace Corporation following a facility inspection which found the company's products, including Calcium/Magnesium Natural Minerals & Trace Elements, Calcium/Magnesium Natural Minerals & Trace Elements with Vitamin D, and Calcium/Magnesium ...
Recalls & Warnings
October 29, 2008
Two Vitamin C Supplements Recalled in Canada for Vitamin A Risk
On October 28, 2008, Health Canada warned Canadians, expecially expectant mothers, not to use two vitamin C products sold under the brand names New Roots Herbal Vitamin C8 and Vitazan Professional Vitamin C Advanced Ascorbate.
Recalls & Warnings
June 19, 2008
FDA Warns Groups to Stop Selling Fake Cancer 'Cures'
On June 17, 2008, the FDA reported that it had sent Warning Letters to 23 U.S. companies and two foreign individuals marketing a wide range of products fraudulently claiming to prevent and cure cancer.
Recalls & Warnings
May 05, 2003
FDA Reports False Claims by Maker of HGH Supplement
On April 30, 2003, the Food and Drug Administration (FDA) announced that Nature's Youth, LLC, of Centerville, Mass., has completed its voluntarily destruction of approximately 5700 boxes (each containing a 30-day supply) of its misbranded product, "Nature's Youth hGH.
Recalls & Warnings
July 03, 2018
FDA Warns Seller of Adulterated Chinese Herbal Supplements
On June 20, 2018, the FDA issued a warning letter to KPC Products, Inc.
Recalls & Warnings
June 05, 2018
FDA Warns Seller of Colloidal Silver
On May 17, 2018, the FDA issued a warning letter to Silver Armor, Inc.
Recalls & Warnings
June 02, 2018
FDA Warns Seller of Cough Suppressant & Grape Seed Supplements
On March 29, 2018, the FDA issued a warning letter to Yoder's Good Health Products following a facility inspection which found a number of the company's products, including Respiratory & Cough Formula and Life Drops to be adulterated because they were prepared, packed, or ...
Recalls & Warnings
April 17, 2018
FDA Warns Seller of Echinacea, Iron, Aloe Supplements & More for Manufacturing Violations
On March 30, 2018, the FDA issued a warning letter to Ozark Country Herbs following a facility inspection which found a number of the company's products, including Echinacea-Goldenseal, Natural Iron, and Aloe-Vera Goldenseal Salve, to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
April 17, 2018
Seller of Osha and Cayenne Supplements & More Warned for Manufacturing Violations
On March 21, 2018, the FDA issued a warning letter to Secret Garden of Health & Healing, LLC, following a facility inspection which found a number of the company's products, including Osha Root and Cayenne, to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
March 31, 2018
Supplement Manufacturer Shut Down for Manufacturing Violations
On March 29, 2018, the FDA announced that U.S. District Court for the Eastern District of New York has entered a consent decree of permanent injunction with Riddhi USA, Inc. and Mohd M. Alam, president and owner of Riddhi USA, Inc., for selling adulterated and misbranded dietary supplements.
Recalls & Warnings
January 13, 2018
Seller of Depression, Prostate Supplements & More Warned for Manufacturing Violations, Drug Claims
On August 21, 2017, the FDA issued a warning letter to Irmo-Vita, LLC, following a facility inspection which found that several of the company's products, including Hysta-Min, Man-Affirm, Phung-EX, and Bacto- EX to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
December 26, 2017
Seller of BrainAlert Warned for Manufacturing Violations, Drug Claims
On December 14, 2017, the FDA issued a warning letter to BrainAlert, LLC.
Recalls & Warnings
December 26, 2017
Seller of Fish Oil, Vitamin D, Whey Protein and More Warned for Manufacturing Violations
On December 19, 2017, the FDA issued a warning letter to Maine Natural Health, Inc.
Recalls & Warnings
October 18, 2017
Total Body Nutrition Warned for Banned Stimulant in Supplements
On September 28, 2017, the FDA issued a warning letter to Total Body Nutrition following a facility inspection which found some of the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
September 28, 2017
Seller of Prostate, Reishi Supplements Warned for Manufacturing Violations
On September 11, 2017, the FDA issued a warning letter to Vicare International (USA), Inc.
Recalls & Warnings
August 29, 2017
FDA Warns Seller of Supplements for Allergies, Joint Pain, Bone Health, and More
On August 16, 2017, the FDA issued a warning letter to Total Nutrition, Inc.
Recalls & Warnings
June 27, 2017
Seller of Alpha Lipoic Acid, Cinnamon Supplements and More Warned For Manufacturing Violations
On May 1, 2017 the FDA issued a warning letter to Nature's Vision, Inc.
Recalls & Warnings
February 16, 2017
U.S. Department of Justice Files Permanent Injunction Against Supplement Manufacturer
On February 16, 2017, the United States Department of Justice filed a complaint against Pick and Pay, Inc.
Recalls & Warnings
September 27, 2016
Seller of B Vitamins, Omega-3s and More Warned for Manufacturing Violations, Drug Claims
On September 15, 2016, the FDA issued a warning letter to Positive Power Nutrition, following a facility inspection which found the company's products, including High Energy C-Complex, Positive Vitality, Positive Performance, Positive CardioGuard, B-Complex 100, Positive Essentials, Positive ...
Recalls & Warnings
September 14, 2016
Seller of Aloe, Prostate and Joint Supplements Warned for Manufacturing Violations
On April 8, 2016, the FDA issued a warning letter to Salud Natural Entrepreneurs, Inc.
Recalls & Warnings
March 08, 2016
Seller of Echinacea Warned Manufacturing Violations, Drug Claims
On February 25, 2016, the FDA issued a warning letter to Herbal Energetics/ In Joy Organics, following a facility inspection which found the company's product, X Out-Rays to be adulterated because it was prepared, packed, or held under conditions that violate Current Good ...
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
June 03, 2015
Maker of B-12 Energy Supplement Warned for Manufacturing Violations
On May 15, 2015, the FDA issued a warning letter to LiquidCapsule Manufacturing, LLC.
Recalls & Warnings
May 02, 2015
Maker of Garcinia, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On March 25, 2015, the FDA issued a warning letter to JW Nutritional LLC, following a facility inspection which found the company's products, including Vanish V2 Domestic, Mr.
Recalls & Warnings
January 17, 2015
Maker of Magnesium and Potassium Supplements Warned for Manufacturing Violations
On December 23, 2014, the FDA issued a warning letter to Nutri Spec, Inc.
Recalls & Warnings
December 30, 2014
Maker of Probiotic Supplement Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to Wellmill LLC, dba Vitamix, following a facility inspection which found the company's products, including Butcher's Broom powder and Lactobacillus acidophilus powder (used as ingredients in a finished product which was not named) to be ...
Recalls & Warnings
December 30, 2014
Maker of Aloe, Weight Loss Supplements and More Warned for Manufacturing Violations, Drug Claims
On December 17, 2014, the FDA issued a warning letter to Dandy Day Corporation, following a facility inspection which found the company's products, including Aloe Pearl, Alfalo, Aloespring, Crave Away, Original Supreme Energy, Ener "G," Can G, Flora G, Flora G Plus, and Garolic, to be adulterated ...
Recalls & Warnings
June 07, 2013
Maker of Colloidal Silver and Mushroom Extract Supplements Warned For Manufacturing Violations and Drug Claims
On May 2, 2013, the FDA issued a warning letter to Earthborn Products, Inc.
Recalls & Warnings
May 10, 2013
Seller Of Sexual Enhancement and Hormonal Balance Supplements Warned For Manufacturing Violations
On April 26, 2013, the FDA issued a warning letter to Pristine Bay, L.L.C.
Recalls & Warnings
November 11, 2014
Maker of Children's Vitamins, Weight Loss Supplements and More Warned for Manufacturing Violations
On October 30, 2014, the FDA issued a warning letter to VitalHealth Tech, Inc., following a facility inspection which found the company's products, including Kids Mighty Vites tablets, Omni Jr.
Recalls & Warnings
October 16, 2014
Seller of Black Cohosh, Ginkgo and More Warned For Manufacturing Violations and Drug Claims
On April 2, 2014, the FDA issued a warning letter to Pure Herbs, Ltd.
Recalls & Warnings
April 02, 2004
Makers of Fat and Starch "Blockers" Receive FDA Warning Letters
On April 1, 2004, the Food and Drug Administration (FDA)announced that it had sent warning letters to 16 dietary supplement distributors making false and misleading claims for weight loss products promoted over the internet.
Recalls & Warnings
June 14, 2007
Marketers of Bogus Growth Hormone Sprays Settle with FTC
On May 29, 2007 the Federal Trade Commission (FTC)announced that two operations that marketed oral sprays that were supposed to help users lose weight, reverse the aging process, and prevent or treat diseases have settled FTC charges that their claims were bogus.
Recalls & Warnings
April 19, 2007
Recall of Another Sex Enhancement Supplement
On April 17, 2007 the Food and Drug Administration (FDA) website posted an announcement that Jen-On Herbal Science International, Inc. is conducting a voluntary nationwide recall of the company's supplement product sold under the name H S Joy of Love. Jen-On Herbal Science International, Inc.
Recalls & Warnings
October 18, 2005
FTC Stops False Claims about HGH Oral Spray
On October 18, 2005, the Federal Trade Commission (FTC) announced that, at its request, a federal court issued a temporary restraining order against marketers of oral sprays that supposedly contain human growth hormone (HGH) to stop them from making alleged false and deceptive claims and from ...
Recalls & Warnings
October 12, 2005
Supplement Company to Pay $2 Million Penalty For Alleged Violations of FTC Order
On October 12, 2005 the Federal Trade Commission (FTC) announced that under the terms of a consent decree approved by the it for submission by the U.S. Department of Justice (DOJ) to the federal court for approval, NBTY, Inc. (NBTY, formerly Nature’s Bounty, Inc.
Recalls & Warnings
July 28, 2010
Choking Concern Prompts Eye Supplement Recall
On July 28, 2010, the U.S. FDA posted a recall by Bausch + Lomb of its PreserVision® Eye Vitamin AREDS 2 Formula with Omega 3 soft gels, only available within the United States.
Recalls & Warnings
October 03, 2014
Seller of Green Tea, Prostate, Pain Supplements and More Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to AMS Health Sciences, LLC following a facility inspection which found the company's products, including saba ACE, UROPOWER, UROSure, Digest-Eze, Shark Cartilage, Colloidal Silver, and Mobilite to be adulterated because they were prepared, packed, ...
Recalls & Warnings
September 17, 2003
FDA Warns of Illness from Star Anise Teas
On September 10, 2003, the Food and Drug Administration (FDA) advised consumers not to consume "teas" brewed from star anise.
Recalls & Warnings
January 11, 2002
Canada Requests Recall of Certain Ephedra/Ephedrine Products
OTTAWA - January 10, 2002 - Health Canada is requesting a recall from the market of certain products containing Ephedra/ephedrine after a risk assessment concluded that these products pose a serious risk to health.
Recalls & Warnings
April 11, 2008
Twelve Dietary Herbal Supplements Recalled -- Possible Health Risk Associated with Ephedra, Aristolochic Acid and Human Placenta
On April 10, 2008, the FDA posted a recall notice issued the same day from Herbal Science International, Inc. (AKA Jen-On Herbal Science International, Inc.).
Recalls & Warnings
October 05, 2007
FTC Charges Progesterone Cream Sellers with Making Unsubstantiated Claims
On October 5, 2007 the Federal Trade Commission (FTC) announced complaints against seven online sellers of alternative hormone replacement therapy (HRT) products, alleging that they made health claims for their natural progesterone creams without supporting scientific evidence.
Recalls & Warnings
June 04, 2004
FTC Charges Marketers of Two Supplements with False Claims to Cure Range of Diseases
On June 3, 2004 the Federal Trade Commission (FTC) charged marketers of two dietary supplements with falsely claiming that their products can prevent and cure cancer and other diseases. According to the FTC’s complaint, Boston-area marketers Direct Marketing Concepts, Inc. (DMC), ITV Direct, Inc.
Recalls & Warnings
December 08, 2011
HCG Diet Products Don't Work and Are Illegal Says FDA
On December 6, 2011, the U.S. FDA warned consumers to avoid homeopathic HCG weight-loss diet products because they don't work, often make unsupported claims, and are illegal for sale. In addition, some of these products direct user to follow a potentially dangerous diet.
Recalls & Warnings
February 03, 2015
Major Retailers Accused of Selling Adulterated Herbal Supplements
On February 3, 2015, the New York State Attorney General announced that his office sent letters to four major retailers, GNC, Target, Walmart, and Walgreens, for allegedly selling store brand herbal supplement products in New York that either could not be verified to contain the labeled substance, ...





