Showing Results for Joint Health
Search term may appear only in full report available to members. Join now for full access.
Product Review
Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, Boswellia, Collagen and Turmeric)
One-Third of Joint Health Supplements Failed to Pass Our Tests. See Which Passed or Failed, and our Top Picks.
Product Review
Joint Health Supplements for Pets Review (Glucosamine, Chondroitin, MSM & Boswellia)
See Which Pet Supplements for Joint Health Passed Our Tests and Which Didn't!

CL Answer
What are the side effects of glucosamine and chondroitin?
Learn the side effects of glucosamine and chondroitin supplements for joint health and joint pain, including stomach upset, headache, and many others. ConsumerLab.com's answer explains.

CL Answer
Does Arthrozene help with joint pain, and is it safe?
Arthrozene by Fisico is promoted for joint stiffness, soreness and discomfort. Find out if it is likely to help and if it is worth the cost.

CL Answer
What is BPC-157, what is it used for and is it safe?
Find out about BPC-157, including what it's promoted for and if it is safe.
CL Answer
Is hyaluronic acid helpful for osteoarthritis?
Hyaluronic acid supplement information including joint pain, osteoarthritis, clinical evidence, dosage and more.

CL Answer
Does taking curcumin and boswellia together increase their individual anti-inflammatory effects?
Find out if taking boswellia and curcumin together can increase their individual anti-inflammatory effects. ConsumerLab.com's answer explains.

CL Answer
My dog is getting older and his veterinarian recently recommended giving him a glucosamine supplement for his joints. Has ConsumerLab.com tested these, or other supplements for pets?
Find out our test results on glucosamine for dogs and cats as well as other supplements for pets.

CL Answer
I have to be careful about my sodium intake. Could my glucosamine and chondroitin supplement contain hidden sodium?
Find out if glucosamine and chondroitin supplements contain hidden sodium and why.

CL Answer
Does collagen taken as a supplement help with arthritis? I'm seeing it in products for joint health.
Learn more about collagen supplements, including evidence from clinical studies on arthritis and muscle strength and safety.

CL Answer
What is UC-II and does it help joints?
What is UC-II (undenatured type 2 collagen - InterHealth Nutraceuticals) and do clinical studies show that it works for joint health?

News Release
February 26, 2024
Latest ConsumerLab Survey Shows Growth in Popularity of Magnesium and Several Smaller Supplements
White Plains, New York, February 26, 2024 — A recent survey of more than 10,000 people who regularly use dietary supplements shows that supplements that experienced the greatest absolute growth in popularity during 2023 were pregnenolone (+7.5 percentage points), magnesium (+4.8 pts), berberine (+4.
CL Answer
Which supplements help for osteoarthritis of the hip?
Supplements for hip pain and osteoarthritis, such as SAMe, ginger and collagen, may help or reduce pain.
CL Answer
Does glucosamine raise "bad" LDL cholesterol?
Learn more about the link between glucosamine and cholesterol, including evidence from clinical studies.

CL Answer
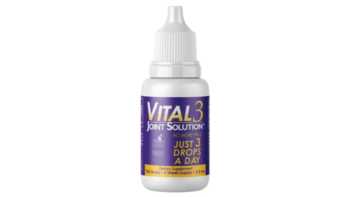
Do Vital 3 collagen drops reduce joint pain?
Learn more about Vital 3 collagen drops withundenatured type II collagen, and if they can help joints move better and feel better.
Clinical Update
6/02/2013
Glucosamine May Increase Eye Pressure
New research suggests that people with glaucoma or intraocular hypertension could have worsening eye pressure if they take a glucosamine supplement. Get the details (along with our tests of glucosamine and other joint health supplements) in the updated Glucosamine/Joint Health Supplements Review >>
Clinical Update
6/13/2014
Contaminated Chondroitin
A chondroitin ingredient on the market has been found to be contaminated with an inexpensive industrial compound. Get the details, along with our tests and comparisons of glucosamine and chondroitin supplements for joint health, in the update to the Review of Glucosamine, Chondroitin, MSM and Boswellia Supplements for Joint Health>>
Clinical Update
3/09/2019
Boswellia for Osteoarthritis
Boswellia extract may reduce the pain and stiffness of knee osteoarthritis. See results of a recent study in the What It Does section of the Joint Health Supplements Review. Also see our Top Picks for Boswellia and other joint health supplements.
Clinical Update
5/15/2019
Glucosamine and Heart Disease
Does taking glucosamine affect your risk of developing cardiovascular disease? See what a large study recently found in the What It Does section of the Joint Health Supplements Review (Glucosamine, Chondroitin, MSM and Boswellia). Also see our Top Picks among joint health supplements.
Clinical Update
3/03/2020
Do Glucosamine & Chondroitin Work?
After reviewing the clinical literature regarding glucosamine and chondroitin for osteoarthritis, an expert group drew conclusions regarding their efficacy. Find out what they concluded in the What It Does section of the Joint Health Supplements Review. Also see our Top Picks for glucosamine, chondroitin and other joint health supplements.
Clinical Update
8/30/2019
Glucosamine for Hand Osteoarthritis?
A new study suggests that glucosamine may help people with hand osteoarthritis. Get the details in the What It Does section of the Joint Health Supplements Review. Also see our Top Picks for joint health supplements.
Clinical Update
3/01/2022
Glucosamine and Depression
Can taking glucosamine improve, or cause, depression? Find out in the Glucosamine and Chondroitin section of our Joint Health Supplements Review. Also see our Top Picks among joint health supplements.
Also see our answer to the question: What are the best supplements for depression and anxiety?
Clinical Update
6/04/2024
Caution With White Willow in Joint Supplements
A CL member asked about potential side effects of taking white willow. Be aware that white willow extract is added to some joint health supplements and can have side effects. For details, see the Concerns and Cautions section of our Joint Health Supplements Review, which includes our Top Picks among products.
Clinical Update
7/12/2024
Glucosamine & Cancer Risk?
Is the use of glucosamine associated with the risk of developing cancer? See what a recent study found in the What It Does section of our Joint Health Supplements Review. Also see our Top Picks for glucosamine and other joint health supplements.
News Release
February 25, 2024
Top-rated Vitamin and Supplement Brands and Merchants for 2024 Based on Consumer Satisfaction
White Plains, New York, February 25, 2024 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 10,000 responses collected in November/December 2023.
News Release
December 28, 2023
10 Most Popular ConsumerLab Product Reviews of 2023
White Plains, New York, December 28, 2023 — In 2023, ConsumerLab tested more than two hundred products, adding to its extensive Product Reviews covering 150 categories of supplements, foods, and other health products. Among these, the following 10 were the most popular in 2023:
CL Answer
Are vitamins and supplements from Canada of better quality because they are licensed?
Find out if Canadian vitamins and supplements are better quality because they are licensed, what it means to for a product to have an Natural Product Number (NPN) in Canada, and if vitamins and supplements in Canada are tested for quality and purity. ConsumerLab.com's answer explains.

CL Answer
Is bromelain a good alternative to NSAIDs, like ibuprofen, for muscle or joint pain?
Do bromelain supplements have benefits? Learn more about its safety, and if it is a good alternative to NSAIDs like ibuprofen.

CL Answer
Which supplements help with arthritis?
Learn more about supplements for joint health, including products such as Wobenzym and Zyflamend and ingredients such as collagen, curcumin, glucosamine and chondroitin, hyaluronic acid, magnesium, omega-3 fatty acids, palmitoylethanolamide (PEA), SAMe, and others.

CL Answer
Which supplements should be taken with food?
Find out which supplements should be taken with food, including vitamins A, D, E, and K, fish oil, and CoQ10.

News Release
July 26, 2023
Best Collagen Supplements for Skin and Joints, According to ConsumerLab Tests
White Plains, New York, July 26, 2023 — Collagen supplementation may modestly reduce wrinkles and the appearance of cellulite, and moderately decrease joint and tendon pain and improve flexibility in people with osteoarthritis.
News Release
February 25, 2023
Top-rated Vitamin and Supplement Brands and Merchants for 2023 Based on Consumer Satisfaction
White Plains, New York, February 25, 2023 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,600 responses collected in November/December 2022.
CL Answer
What is Omega XL and does it work?
What is Omega XL (distributed by Great Health Works) and does it work? Described as a "highly concentrated Omega-3 'Super Oil' supplement".
Product Review
Maca Supplements Review
Choose the Best Maca Supplement. Make Sure Your Maca Supplement Isn't Contaminated With Lead.

Product Review
Collagen Supplements Review
See our Top Picks for Wrinkles and Joints

Clinical Update
9/07/2019
Vegetarian Chondroitin for Knee Pain?
Can "vegetarian" chondroitin reduce knee pain and improve mobility? See what a recent study found in the What It Does section of the Joint Health Supplements Review. Also see our Top Picks for glucosamine and chondroitin supplements.
Clinical Update
12/07/2019
Glucosamine & Chondroitin – How They May Work
A recent study suggests that one way glucosamine and chondroitin may work is through the gut. For details, see the What It Does section of the Joint Health Supplements Review (Glucosamine, Chondroitin, MSM and Boswellia). Also see our Top Picks among products.
Clinical Update
2/08/2020
Glucosamine and Blood Sugar
Glucosamine (used to treat osteoarthritis) is a type of sugar, so does its daily use affect blood sugar levels and one's risk of developing diabetes? Find out in the Concerns and Cautions section of the Joint Health Review. Also see our Top Picks for Glucosamine, Chondroitin, MSM, and Boswellia supplements.
Clinical Update
7/23/2017
Does MSM Help Joints and Muscles?
MSM (methylsulfonylmethane) is commonly in supplements for joint and muscle pain, although the evidence behind it has been limited. Its effectiveness in reducing pain, muscle damage, and oxidative stress was recently evaluated in people running a half-marathon. See the results in the "What It Does" section of the Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, and Boswellia), which includes our Top Picks among products.
Clinical Update
5/28/2019
Glucosamine & Eye Pressure
Glucosamine may increase eye pressure in people with glaucoma or intraocular hypertension. But does it have this effect in people who don't have these conditions? See what a new study found in the Concerns and Cautions section of the Joint Health Supplements Review. Also see our Top Picks for glucosamine and chondroitin.
Clinical Update
4/26/2016
Knee Supplements Studied
A recent clinical trial evaluated glucosamine with chondroitin versus placebo for reducing pain and improving function in people with osteoarthritis of the knee. See the details, along with results of our tests of popular supplements, see our Review of Glucosamine, Chondroitin, MSM and Boswellia Supplements for Joint Health >>
Clinical Update
1/09/2018
Curcumin for Knee Osteoarthritis
A type of curcumin that has been Approved for Quality by ConsumerLab.com was found to be modestly effective in treating symptoms of knee osteoarthritis in a recently published study. For details see the Osteoarthritis section of the Turmeric & Curcumin Supplements Review.
The same study found that combining curcumin with boswellia may be advantageous. Learn more about this and other popular supplements for joint health in the Joint Supplements Review >>
Clinical Update
9/25/2011
Chondroitin Improves Hand Arthritis
Do you or someone you know have osteoarthritis of the hand? A new study found that highly purified chondroitin can reduce pain and stiffness and increase function. You need to give it some time, though. Get the details in the update in the Joint Health Supplements Review. More >>
Clinical Update
2/23/2022
Boswellia for Knee Pain?
Can Boswellia extract reduce knee pain and stiffness in people with knee osteoarthritis? See what a recent study found in the What It Does section of our Joint Health Supplements Review. Also see our Top Picks among products containing Boswellia, glucosamine, chondroitin, and/or MSM.
See our answer to the question: Which supplements can help with arthritis?
Clinical Update
6/10/2022
Top Pick for Boswellia (Anti-Inflammatory)
We just tested two Boswellia extract supplements (containing the anti-inflammatory compound AKBA). One failed testing but the other passed and is now our Top Pick for Boswellia. The results are in our Joint Health Supplements Review, which include our tests of supplements with glucosamine, chondroitin, MSM and other ingredients.
Clinical Update
7/12/2022
Hyaluronic Acid Injections for Knee Pain?
Do hyaluronic acid injections reduce pain or improve function in people with knee osteoarthritis? Find out what a recent analysis showed in the Hyaluronic acid section of our Joint Health Supplements Review.
Clinical Update
9/13/2022
Glucosamine & Heart Disease
Is taking glucosamine for arthritis linked to the risk of cardiovascular disease? Find out in the What It Does section of our Review of Joint Health Supplements.
Clinical Update
9/30/2022
Does MSM Help Hair and Nails?
Find out if research supports MSM (methylsulfonylmethane) for improving nails or hair in the MSM section of our Joint Health Supplements Review.
Also see our article about biotin and other supplements for hair and nails.
Clinical Update
6/27/2023
Does Omega XL Work?
We’ve updated our article about this supplement widely promoted for joint health and muscle recovery. See our updated information about Omega XL.
Clinical Update
10/17/2023
Citrus Pectin for Knee Pain?
Can modified citrus pectin (MPC) reduce inflammation or pain in people with knee osteoarthritis? Find out what a clinical study showed in our article about supplements for arthritis.
Also see the evidence for other ingredients promoted for osteoarthritis and our Top Pick supplements for joints in our Joint Health Supplements Review.
Clinical Update
11/03/2023
Collagen For Bone Health?
Is there evidence that collagen peptides (as in Fortibone) strengthen bones? Find out in the bone health section of our Collagen Supplements Review. Also see our Top Picks for collagen for skin and joint health.
Also see: Do any supplements or diets help prevent or treat osteoporosis?
Clinical Update
4/26/2024
Medication Found in Umary Supplement
A potent medication was found in Umary Hyaluronic Acid, with serious side effects reported. Get the details. Also see our Top Picks among supplements for joint health.
Clinical Update
1/23/2024
Hyaluronic Acid for Joint Pain?
Did taking hyaluronic acid reduce pain and improve function in people with knee pain in a recent study? Find out in the Hyaluronic acid section of our Joint Health Supplements Review. Also see our Top Picks among supplements for joints.
CL Answer
I was told I have a MTHFR gene mutation. Do I need to take methylated forms of B vitamins, such as methylfolate and methylcobalamin?
MTHFR gene mutation can impact B vitamin bioavailability. Learn more about this and which deficiencies are more likely to occur.

Recalls & Warnings
November 28, 2022
Seller of Joint Health, Collagen Protein and More Warned for Drug Claims
On November 14, 2022, the FDA issued a warning letter to The Truth Company, LLC (parent company of Kinobody, LLC and UMZU, LLC) following inspection of the company’s websites which found statements about its Betaine, Immune, Redwood, Sensolin, Thyrite, zuRelief, Kino Aminos, Kino Collagen ...
Clinical Update
3/08/2014
Glucosamine & Chondroitin Help Maintain Knee Joint
A 2-year study in people with osteoarthritis of the knee found that those who took a combination of glucosamine and chondroitin had only half the amount of narrowing of the knee joint space as those who took placebo. This beneficial finding differs from that of another large study published several years ago which did not find a benefit on joint space. The new study used a different form of glucosamine than the previous study. Get the details, as well as our tests of supplements with these ingredients, in the updated Joint Health Supplements Review (Glucosamine, Chondroitin, MSM and Boswellia) >>
CL Answer
Do glutathione supplements work to prevent aging or for other conditions?
Do glutathione supplements help with aging or other conditions such as cancer and diabetes?
CL Answer
Can bloodroot or "black salve" treat skin cancer? Is it safe?
Bloodroot salve and black salve made from bloodroot and zinc - are they effective and safe treatments for skin cancer? ConsumerLab.com's answer explains.
News Release
February 24, 2023
Probiotics Rise in Popularity as Vitamin C, Melatonin, and Others Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 25, 2023 —A recent survey of 8,600 people who regularly use dietary supplements shows that probiotics (+3.04 percentage points), quercetin (+2.3 pts), and vitamin K (+1.
News Release
February 25, 2022
Top-rated Vitamin and Supplement Brands and Merchants for 2022 Based on Consumer Satisfaction
White Plains, New York, February 25, 2022 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,049 responses collected in November/December 2021.
News Release
February 24, 2022
Consumers Returned to Pre-Pandemic Supplement Usage in 2021, ConsumerLab Survey Reveals
White Plains, New York, February 24, 2022 —A survey of 8,049 people who use dietary supplements shows many supplements that declined in use in 2020 began bouncing back in 2021, such as magnesium (+2.4 percentage points), and CoQ10 (+2.7 pts).
CL Answer
I've read about several different forms of SAMe. Is one form better than another?
Learn about the various types of SAMe (S-adenosyl-L-methionine) supplements, along with their benefits for hormones & cell membrane health.

Recalls & Warnings
March 09, 2022
Court Bars Salud Natural From Selling Aloe, Joint Supplements & More
On March 8, 2022, a federal court ordered Salud Natural Entrepreneur, Inc. to stop distributing nutritional supplements that violate the Federal Food, Drug and Cosmetic Act (FDCA).
CL Answer
Does chuchuhuasi reduce inflammation or back pain?
Find out if chuchuhuasi, an herb from the Amazon rainforest, can decrease inflammation or reduce back pain. ConsumerLab.com's answer explains.

Recalls & Warnings
May 09, 2013
Joint Health Supplement Recalled for Undeclared Allergens
On May 1, 2013, XYMOGEN issued a voluntary recall of the joint health supplement artriphen, because it contains undeclared soy and milk allergens.
Recalls & Warnings
September 28, 2022
FDA Warns Muscle Sports for Joint Health, Immune & Workout Supplement, Vitamin C, Elderberry, and Other Claims
On September 23, 2022, the FDA issued a warning letter to Muscle Sports Products, LLC following inspection of the company’s websites which found statements about company products, including Join Revolution Capsules, IMMUNITY + Powder, Rhino Rampage PUMPED Capsules, Attack Pre-Workout ...
News Release
August 27, 2021
ConsumerLab Tests Reveal Best Joint Health Supplements for People and Pets
White Plains, New York, August 27, 2021 — Joint health supplements with ingredients such as glucosamine, chondroitin, MSM, boswellia, collagen and turmeric are often promoted to relieve joint pain or slow the progression of osteoarthritis.
CL Answer
What are the symptoms of vitamin D deficiency?
Learn about the symptoms of vitamin D deficiency, such as soft or weak bones, bone, joint and muscle pain, depression, insomnia, hair loss and others, and find out how much vitamin D you need. ConsumerLab.com's answer explains.

CL Answer
Do collagen supplements impact cholesterol levels or reduce risk factors for atherosclerosis?
Collagen has been studied for its effects on cholesterol levels and atherosclerosis risk factors, but its effects are still not clear.
Recalls & Warnings
May 01, 2024
FDA Warns Consumers Not to Use Ossos-Sans Supplement for Joints
On April 17, 2024, the FDA warned consumers not to purchase or use Ossos-Sans, a supplement promoted to treat osteoarthritis, osteoporosis, and other conditions, because it contains undeclared drugs, including diclofenac and methocarbamol.
CL Answer
Does urolithin A reduce age-related muscle decline or improve osteoarthritis?
Find out if urolithin A may improve age-related muscle decline and learn about its safety.

Recalls & Warnings
November 09, 2023
Zazzee Naturals Warned for MSM Eye Drop Claims
On October 30, 2023, the FDA issued a Warning Letter to Dexterity Health, LLC DBA Zazzee Naturals following review of the company’s Amazon storefront, which found statements about the company’s Liquid MSM Drops to be drug claims.
CL Answer
What are the health benefits of andrographis? Can it treat colds, help with joint pain, or other conditions, and is it safe?
Learn about the health benefits of andrographis, whether it can help relieve cold symptoms, reduce pain and stiffness of osteoarthritis or rheumatoid arthritis, decrease symptoms of ulcerative colitis, or slow the growth of cancer. See the clinical evidence for the branded andrographis extract ParActin. Plus, learn about dosage, side effects and drug interactions with andrographis.

CL Answer
Are there any serious side effects with Lovaza or Epanova (prescription fish oil)? If so, would these also apply to generic versions of these drugs and to fish oil supplements?
Understand the side effects of prescription fish oil like Lovaza and Epanova, and find out if the generic fish oils are effective and safe.

Recalls & Warnings
October 26, 2023
Joint Pain Supplements Recalled Due to Undeclared Drug Ingredients
On October 22, 2023, Botanical-Be recalled of all lots of the company’s Kuka Flex Forte, Artri King, and Reumo Flex capsule products after FDA laboratory analysis found them to contain undeclared diclofenac.
Recalls & Warnings
May 18, 2023
EarthLab, Inc. Warned for Promoting Green Tea, Curcumin, Elderberry & More to Treat Stroke, Cancer & Flu
On April 27, 2023, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals, following inspection of the company’s website which found statements about the company’s Green Tea Solid Extract, Curcuma Spp.
Recalls & Warnings
September 25, 2018
Seller of B Vitamins, Multis, Glucosamine & More Warned for Manufacturing Violations
On August 31, 2018, the FDA issued a warning letter to Independent Nutrition Inc., following a facility inspection which the company's products, including B-50 Complete, Multi-Vitamin & Mineral Complex (a.k.a.
CL Answer
What are the health benefits of tart cherry juice?
See the evidence for tart cherry juice health benefits from clinical studies. Find out if tart cherry has anti-inflammatory effects, if it can improve sleep, lower high blood pressure, or help for muscle pain and osteoarthritis.

Recalls & Warnings
October 02, 2023
Dangerous Ingredients Found in Ten Arthritis and Pain Supplements
On September 29, 2023, the FDA warned consumers that the following arthritis and pain management products contain undeclared drugs such as diclofenac, methocarbamol (a muscle relaxant), and dexamethasone (a corticosteroid), and/or other potentially harmful ingredients.
Recalls & Warnings
June 26, 2024
FDA Warns Consumers Not to Use Infla-650 Due to Undeclared Drugs
On June 20, 2024, the FDA warned consumers not to purchase or use Infla-650 after FDA laboratory analysis found them to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
July 16, 2024
Infla-650 Herbal Dietary Supplement Recalled
On July 16, 2024, Guru Inc. issued a recall of one lot of Infla-650 Herbal Dietary Capsules because they were found to contain acetaminophen, diclofenac, and phenylbutazone.
Recalls & Warnings
July 22, 2024
Umary Hyaluronic Acid Supplement Recalled
On July 15, 2024, SoloVital.
CL Answer
What are the health benefits of serrapeptase?
Serrapeptase is a proteolytic enzyme. Learn more about its health effects, including whether evidence supports its use for pain, inflammation, and heart health.

Recalls & Warnings
February 11, 2020
FTC Sues Two Companies Selling Bone Health and Joint Pain Supplements
On February 11, 2020, the Federal Trade Commission (FTC) announced that it sued two companies to stop them from continuing to make false claims about their bone and joint health products, Ostinol (ZyCal Bioceuticals) and StimTein (Excellent Marketing Results, Inc. (EMR)).
Recalls & Warnings
September 03, 2019
Federal Court Shuts Down Two Supplement Companies Selling Weight, Joint Health and Other Supplements
On September 3, 2019, a U.S. District Court entered a consent decree of permanent injunction against Basic Reset and Biogenyx, two Tennessee-based companies that sell dietary supplements and other product promoted for health benefits.
Recalls & Warnings
January 15, 2024
Suprex Carb & Sugar Block Recalled
On December 7, 2023, Vita 360, LLC issued a recall of one lot of SUPREX Plant Based Nutrition Carb & Sugar Block after FDA analysis found it to contain only 16 mcg of chromium per serving and not 100 mcg of chromium per serving, as listed on the label.
Recalls & Warnings
August 08, 2022
Woman Pleads Guilty for Selling and Promoting Products to Treat COVID-19
On July 27, 2022, Diana Daffin, owner of Savvy Holistic Health pleaded guilty to selling products with claims they could treat COVID-19 and promised “Immunity for Humans.” The products were sold under the HAMPL brand name.
Recalls & Warnings
April 25, 2024
Drug Found in Umary Supplement
On April 18, 2024, CTV News in Canada reported tests suggesting that Umary Hyaluronic Acid Dietary Supplement may be adulterated with the prescription anti-inflammatory drug diclofenac, which may explain side effects that have been reported with its use.
Recalls & Warnings
November 29, 2023
Dr. Berne’s Whole Health Products Warned for Contaminated Eye Drop Products, Drug Claims
On November 22, 2023, the FDA issued a Warning Letter to Dr. Berne’s Whole Health Products following review of the company’s website, which found statements about the company’s Dr. Berne’s MSM Eye Drops 5%, Dr. Berne’s MSM Eye Drops 15%, and Dr.
Recalls & Warnings
January 10, 2024
Spring Valley Biotin & Collagen Liquid Recalled Due to Mold
On December 1, 2023, BioMylz Pvt. Ltd. issued a recall of 24,324 units of Spring Valley Biotin & Collagen Liquid Natural Berry Flavor products due to potential mold contamination. BioMylz is the contract manufacturer for the Spring Valley product, which is a Walmart-owned brand.
CL Answer
Do any supplements or diets help prevent or treat osteoporosis?
If you have osteoporosis, you may be interested in supplements or diets for bone health. Find out which can increase bone strength and density.

CL Answer
Does cat’s claw have any health benefits and is it safe?
Cat's claw is promoted for numerous conditions, including arthritis (including osteoarthritis and rheumatoid arthritis), Alzheimer's disease, viral infections, boosting immune response to vaccination, and reducing side effects of chemotherapy. Find out if it works and if it's safe.

Recalls & Warnings
November 21, 2022
Healthy Sense and People’s Choice Multis Recalled
On November 17, 2022, Mason Vitamins Inc.
CL Answer
Do any supplements help treat or reduce the pain of plantar fasciitis (heel pain)?
Plantar fasciitis causing heel pain has, in rare cases, been reported as a symptom of vitamin D deficiency. Other supplements that have been proposed to help include vitamin C, zinc, curcumin (from turmeric), bromelain, collagen and glucosamine. Find out what the evidence shows, and what other treatments may help.

Recalls & Warnings
September 08, 2020
Holistic Healthy Pet Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Savvy Holistic Health dba Holistic Healthy Pet following a review of the company's website, which found statements made about some of the company's products, including Doctor's Best for Your Pets, Savvy Holistic Health Pet Essences, ...
News Release
February 26, 2021
COVID Changed Supplement Popularity in 2020, ConsumerLab Survey Reveals
White Plains, New York, February 26, 2021 — A survey of 9,647 people who use dietary supplements shows that the supplements which experienced the greatest growth in popularity in 2020 were those being promoted to prevent or treat infection with SARS-CoV-2, the coronavirus that causes COVID-19.
CL Answer
3 Tips to Prevent Powdered Supplements from Clumping
Learn more about how powdered supplements compare to capsules and if clumping is worrisome.

Recalls & Warnings
July 31, 2018
FDA Warns Seller of Joint Health and Cholesterol Supplements
On July 13, 2018, the FDA issued a warning letter to GC Natural, following a facility inspection which found a number of the company's products, including Red Pyrola Plus + Advanced Joint Formula Capsules, CSDP GOLD Capsules, Rejeune Optimal Kidney & Liver Support Extract balls, C.
Recalls & Warnings
September 19, 2018
Seller of Curcumin, Garcinia, Glucosamine and More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to Best Nutrition Products, Inc.
Recalls & Warnings
February 22, 2024
Pacific BioLogic Co. Warned by FDA for Manufacturing Violations
On December 21, 2023, the FDA issued a Warning Letter to Curtis Jacquot, dba Pacific BioLogic Co.
CL Answer
Does red and near infrared light therapy reduce pain, improve skin or cognition, or have other benefits? Is it safe?
Red light and near infrared light therapy devices are promoted for numerous conditions, including acne, fibromyalgia, pain, cognitive function and other uses. Find out if these devices work and if they are safe.

Recalls & Warnings
November 28, 2022
Seller of Elderberry, Tea Warned for Claims of Treating Cold, Flu, Cancer
On October 18, 2022, the FDA issued a warning letter to Rosebud’s Ranch and Garden, LLC after inspection of the company’s website and social media found statements about the company’s Domestic Divas – Colds and Flu (Tea), Domestic Divas - No Pain No Gain Tea, Green ...
Recalls & Warnings
December 29, 2022
EarthLab, Inc. Warned for Promoting Curcumin, Elderberry to Treat Pain & Flu
On November 10, 2022, the FDA issued a warning letter to EarthLab, Inc., dba Wise Woman Herbals following inspection of the company’s website which found statements about the company’s products to be drug claims because they suggest the products can prevent or treat disease.
Recalls & Warnings
July 26, 2023
Five Leaf Pet Botanicals Warned by FDA for Drug Claims
On June 22, 2023, the FDA issued a warning letter to Five Leaf Pet Botanicals, Inc.
Recalls & Warnings
April 03, 2024
FDA Warns Ambaya Gold for Promoting Products for Depression, Cancer, & Arthritis
On December 5, 2023, the FDA issued a Warning Letter to Ambaya Gold Health Products, LLC following review of the company’s website and social media, which found statements about the company’s Brain Balance, Immune System Boost, Dentist In A Bottle, Essensiac, Fulvic Green, Silver ...
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
CL Answer
COVID-19 Vaccines for 2023-2024: What You Should Know
Learn how the updated COVID-19 vaccines for 2023-2024 (Moderna, Novavax, and Pfizer-BioNTech) differ from each other, who should get them, what the recommended dosing schedules are, and possible side effects.
CL Answer
Does silica have health benefits? What is BioSil, and can it really increase collagen production and strengthen hair, skin, nails and bones?
Information about the health effects of silica (silicon dioxide) and orthosilicic acid (an ingredient in BioSil), including whether these forms of silicon can really increase collagen production, thicken hair, strengthen nails & bones.
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
Recalls & Warnings
December 08, 2020
FTC Sends Refund Checks to Consumers of Deceptive Joint Pain Supplement Synovia
On December 1, 2020, the FTC announced it is mailing 13,221 checks totaling nearly $775,000 to consumers who bought Synovia, a supplement intended to treat joint pain and arthritis.
News Release
February 25, 2021
Top-rated Vitamin and Supplement Brands and Merchants for 2021 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2021 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,647 responses collected in November 2020.
Recalls & Warnings
January 16, 2014
Joint Health Supplement Contains Dangerous Drugs, FDA Warns
On January 15, 2014, the FDA warned consumers not to buy or use joint health supplement Pro ArthMax (by Human Science Foundation) because it was found to contain multiple drugs, including diclofenac, ibuprofen, naproxen, indomethacin, nefopam, and chlorzoxazone.
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Turmeric, Ashwagandha, Glucosamine, and More
On December 18, 2020, the FDA issued a warning letter to Reddy Naturals following a review of the company's website, which found statements made about the company's products Glycostasis, Glucosamine Curcumin, Turmeric 50, Turmeric Curcumin, Cetyl Curcumin, Ashwagandha, Huruli, ...
Recalls & Warnings
December 15, 2022
Beware of Supplements, Homeopathic Products Promoted to Treat Flu, Says FDA
On December 13, 2022, the FDA warned consumers to beware of supplements and homeopathic products, as well as nasal sprays, sanitizers and other products that promise to prevent or treat the flu.
News Release
February 25, 2020
Top-rated Vitamin and Supplement Brands and Merchants for 2020 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2020 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,782 responses collected in late November and early December 2019.
Recalls & Warnings
July 14, 2022
FDA Warns Company Selling Supplements with Dangerous Steroid-like Substances
On July 6, 2022, the FDA issued a warning letter to Elite Supplement Center LLC and Elite Supplement Training Facility LLC for selling the following products labeled as containing steroid-like substances known as selective androgen receptor modulators (SARMs), which are not permitted in dietary ...
Recalls & Warnings
January 12, 2021
FDA Warns Seller of Joint and Hair Loss Products
On December 22, 2020, the FDA issued a warning letter to Speedwinds Nutrition, Inc. following a review of the company's website, which found statements made about the company's products Synotrex and Sephren to be drug claims.
Recalls & Warnings
December 09, 2021
FDA Warns Seller of Curcumin, Lion's Mane, Quercetin & More
On November 9, 2021, the FDA issued a warning letter to Synaptent, LLC because it found statements on the company's website about its products, including Berberine HCL, Curcumin, Lion's Man, Milk Thistle, Quercetin, Boswellia Serrata Extract, and Garlic Extract to be ...
News Release
October 04, 2019
ConsumerLab Tests Reveal Best Collagen Supplements for Wrinkles and Joints
White Plains, New York, October 4, 2019 — Collagen supplements are promoted to reduce wrinkles and decrease joint pain and stiffness, but do they really work, and if so, which products on the market provide the best quality collagen and the best value? To find out, ConsumerLab carefully ...
Recalls & Warnings
March 30, 2019
FDA Warns Seller of Reishi, Joint Supplements, Ginseng & More for Pesticides, Other Violations
On March 12, 2019, the FDA issued a warning letter to Yanqing "Michael" Li and Chang Su, owners of the website http://www.woohoonatural.
News Release
May 15, 2019
ConsumerLab Tests Reveal Big Differences in Digestive Enzyme Supplements
White Plains, New York, May 15, 2019 — Digestive enzyme supplements can help improve digestion and the absorption of nutrients, and may reduce symptoms of indigestion. But to work, they must provide a certain amount of enzyme activity.
News Release
February 25, 2019
Top-rated Vitamin and Supplement Brands and Merchants for 2019 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2019 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,931 responses collected in late November and early December 2018.
Clinical Update
3/14/2023
Collagen "Boosters"?
A CL Member asked if taking Essential Skin Food -- or other non-collagen products marketed for collagen production -- reduces wrinkles. Find out in the ConsumerTips section of our Collagen Supplements Review.
Also see our Top Pick collagen supplements for skin health and for joint pain.
Recalls & Warnings
January 30, 2018
Limbrel for Osteoarthritis Recalled Due to Risk of Adverse Effects
On January 26, 2018, Primus Pharmaceuticals, Inc. of Scottsdale, Arizona issued a recall of all unexpired lots of Limbrel products.
Recalls & Warnings
January 25, 2018
Bulletproof Collagen Protein Recalled
On January 25, 2018, Bulletproof 360, Inc issued a recall of its Bulletproof Collagen Protein supplement because it contains undeclared milk.
Recalls & Warnings
December 05, 2019
Joint Supplement Synovia Was Promoted With Phony Testimonials, Says FTC
A.S. Research, LLC, has agreed to settle Federal Trade Commission (FTC) charges that they deceived consumers with false claims that their dietary supplement Synovia could treat arthritis and alleviate joint pain.
Recalls & Warnings
November 10, 2020
Seller of Cholesterol-Lowering Supplements Warned for Drug Claims
On October 29, 2020, the FDA issued a warning letter to Natural Sprout Co.
Recalls & Warnings
March 04, 2021
Federal Court Bars Confidence USA from Selling Supplements
On March 4, 2021, the FDA announced a permanent injunction has been entered, barring dietary supplement manufacturer Confidence USA Inc. and two of its executives from producing or selling products until they become compliant with current good manufacturing practices (cGMPs).
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
December 11, 2020
FDA Warns Seller of Curcumin and Cholesterol Supplements
On November 20, 2020, the FDA issued a warning letter to Natures Boost LLC following a review of the company's websites, which found statements made about the company's products Blood Boost Formula and Turmeric Curcumin to be drug claims.
Clinical Update
7/24/2021
Collagen for Joint Pain?
Can collagen supplementation reduce joint pain or stiffness in the knee, hip or wrist? See what a new study found in the Joint Pain section of our Collagen Supplements Review. Also see our Top Picks among collagen supplements for joints and for skin.
Clinical Update
6/30/2023
Vital 3 for Joint Pain?
Does Vital 3 Joint Solution reduce joint pain? Find out in our updated article about Vital 3 Joint Solution.
Recalls & Warnings
September 25, 2018
FDA Warns Seller of Digestive Enzyme Supplements
On March 6, 2018, the FDA issued a warning letter to Uckele Health & Nutrition, Inc.
Recalls & Warnings
October 06, 2020
FDA Warns Seller of Red Yeast Rice, Vitamin D, Blood Pressure Supplements, and More
On August 28, 2020, the FDA issued a warning letter to Dr. Sam Robbins, Inc.
News Release
October 02, 2018
Best Joint Health Supplements Identified -- ConsumerLab Tests Glucosamine, Chondroitin, MSM and Boswellia Supplements for People and Pets
White Plains, New York, October 2, 2018 — Do joint health supplements with ingredients such as glucosamine, chondroitin, Boswellia, and MSM really relieve joint pain or slow the progression of osteoarthritis? If so, which products are best? To find out, ConsumerLab recently tested popular ...
News Release
March 27, 2018
You Can't Always Rely on Bone Broth Labels -- ConsumerLab Tests Reveal How Much Protein, Collagen and Sodium Is Really In Bone Broths
White Plains, New York, March 27, 2018 — Bone broth is promoted as a natural source of collagen, an immune-booster, and a remedy for everything from joint pain to wrinkles.
News Release
February 25, 2018
Top-rated Vitamin and Supplement Brands and Merchants for 2018 Based on Consumer Satisfaction
White Plains, New York, February 25, 2018 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 11,446 responses collected in late November and early December 2017.
Recalls & Warnings
December 19, 2013
Maker of Joint Health Supplements Warned for Manufacturing Violations
On December 4, 2013, the FDA issued a warning letter to PurQuality, LLC.
News Release
February 24, 2017
Vitamin D Supplements Maintain Top Spot in Popularity, as Probiotics Surpass Multivitamins to #4 Behind Fish Oil and CoQ10 in Latest ConsumerLab.com Survey
White Plains, New York, February 24, 2017 — A recent survey of over 9,505 people who use dietary supplements shows the most popular dietary supplement to be vitamin D, followed by fish oil, CoQ10, probiotics, and multivitamins.
Clinical Update
8/14/2020
Eggshell Membrane + Fish Oil for Joint Pain?
Both eggshell membrane and fish oil have been shown to bring modest reduction in joint pain, but does taking these ingredients together have greater benefit? Find out what a new study showed in the What It Does section of the Collagen Supplements Review. Also see our Top Pick among collagen supplements for joints and for skin.
Clinical Update
Clinical Update
3/29/2022
Collagen for Joint Discomfort?
Can supplementing with low-dose collagen reduce joint pain in healthy older adults? Find out what a recent study showed in the Joint Pain section of our Collagen Supplements Review. Also see our Top Picks for collagen.
Recalls & Warnings
April 17, 2020
Arthritis Supplement Recalled for Salmonella Risk
On April 13, 2020, Arthri-D, LLC issued a recall of one lot of Arthri-D arthritis supplement because recent testing found it has the potential to be contaminated with Salmonella.
News Release
July 29, 2016
ConsumerLab.com Discovers Problems with Most Multivitamins for Dogs and Cats
White Plains, New York, August 2, 2016 — After discovering in January that none of the three pet multivitamins it had purchased and tested contained all of what was listed on the labels, ConsumerLab.
News Release
June 03, 2016
What Works for Joint Health? ConsumerLab.com Reviews Glucosamine, Chondroitin, MSM and Boswellia Supplements for People and Pets
White Plains, New York, June 3, 2016 — Do popular joint health supplements with ingredients such as glucosamine, chondroitin, Boswellia, and MSM really work to relieve pain or slow the progression of osteoarthritis? And, with so many to choose from, which supplements are best? To find out, ...
News Release
February 26, 2014
Mislabeling of Some Joint Health Supplements with Glucosamine, Chondroitin, Boswellia, and MSM According to ConsumerLab.com -- 38 Supplements Tested, Including Products for Dogs and Cats --
White Plains, New York, February 26, 2014 — Glucosamine, chondroitin, Boswellia, and MSM are popular dietary supplement ingredients for treating symptoms of osteoarthritis — worn joint cartilage.
Recalls & Warnings
April 14, 2020
Teami Tea Settles Charges of Unproven Claims and Deceptive Celebrity Endorsements
On March 6, 2020, the FTC announced that Teami, LLC, a marketer of tea and skin care products, agreed to settle charges that it was selling products with unproven claims and misleading celebrity endorsements.
Recalls & Warnings
June 02, 2020
Sundial Herbal Ordered to Halt Distribution of Products
On June 1, 2020, a New York federal judge filed an order against two individuals doing business as Sundial Herbal Products to stop the company from distributing its products, which were found to be misbranded and promoted as drugs.
Clinical Update
6/23/2023
Arthrozene for Joint Pain?
Does Arthrozene help relieve joint pain and is it safe? Find out in our new article about Arthrozene.
Clinical Update
8/25/2023
Zyflamend for Joint Pain?
Can taking Zyflamend, a supplement with 10 herbal extracts, help improve symptoms of arthritis or other types of joint pain? Find out what research suggests in our article about supplements for arthritis.
Clinical Update
2/01/2020
Collagen for Knees?
Collagen supplements have been tested in many studies to reduce pain and/or improve function in osteoarthritic knees. A recent study focused on collagen peptides. Find out if they worked (and see the results for other collagen products) in the Joint Pain section of the Collagen Supplements Review. Also see our Top Picks for collagen supplements for joint pain and for wrinkles.
Clinical Update
5/04/2021
PEA for Joint Pain?
Can PEA (palmitoylethanolamide) reduce joint pain? Find out what a recent study found in our answer to the question: Which supplements can help with arthritis?
Recalls & Warnings
June 16, 2020
NutraCap Labs Warned for Unsafe Ingredients and Manufacturing Violations
On May 21, 2020, the FDA issued a warning letter to NutraCap Labs LLC, which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
December 27, 2019
Federal Court Shuts Down Three Supplement Companies With Serious Manufacturing Violations
Update: (1/23/20) ABH has issued a recall of all its products, which are sold under various brand names by over 800 distributors and retailers. More details are available in CL's post about this recall.
Recalls & Warnings
August 21, 2019
Large Multivitamin, Calcium Pills Can Be a Choking Hazard for Older Adults
Approximately 19% of adverse events associated with dietary supplements involve swallowing problems, especially choking, and this is mostly likely to occur in adults age 65 or older, according to a new report published in the Annals of Internal Medicine.
Recalls & Warnings
September 19, 2018
FDA Warns Seller of Shilajit
On September 6, 2018, the FDA issued a warning letter to Adaptive Energy LLC, following an inspection of the company's website, which found statements made about its shilajit product, Purblack were found to be drug claims.
Clinical Update
7/18/2024
Red Light Devices – More Answers
We've added more information to our recent article about red near infrared light devices about based on questions received:
- Vitiligo: Does red light help with vitiligo?
- Eye conditions: Is red light effective and safe for treating eye conditions , such as macular degeneration?
- Joint Pain: Is Move+ Pro by Kineon cleared by the FDA for reducing joint pain?
- Cleared by FDA? What is the difference between a device that has been "cleared" by the FDA as opposed to "approved" by the FDA?
- Product status: What is the FDA status of Omnilux Contour Face Mask, TenDlite, PainAway, and FibroLux?
- Devices vs. Sunshine? Since sunshine includes the wavelengths from red and near infrared light devices, why not just get sun exposure?
News Release
May 08, 2012
Lead Contamination and Mislabeling in Joint Health Supplements with Glucosamine, Chondroitin, and MSM According to ConsumerLab.com
White Plains, New York, May 8, 2012 — Glucosamine, chondroitin, and MSM are popular dietary supplements for treating symptoms of osteoarthritis — worn joint cartilage.
News Release
February 16, 2011
Some turmeric and curcumin supplements fail quality review -- ConsumerLab.com finds 20% of turmeric supplements selected for review deliver less than 15% of promised compounds
WHITE PLAINS, NEW YORK — FEBRUARY 16, 2011 — Supplements containing the herb turmeric or its key compound, curcumin, have become popular in the U.S. ConsumerLab.com cautioned today that two out of ten turmeric products recently selected for quality testing were found to provide only 7.
News Release
July 10, 2009
Nine joint health supplements fail ConsumerLab.com quality review -- Lead contamination, missing ingredients and other problems found in supplements for people as well as for dogs, cats and horses; Many others approved
WHITE PLAINS, NEW YORK — JULY 10, 2009 — Nine supplements for osteoarthritis that ConsumerLab.com recently selected for testing were found to be contaminated with lead, lack all or some of a key ingredient or have other quality problems.
News Release
May 19, 2009
Review of Magnesium Supplements by ConsumerLab.com Shows One-Quarter Fail Quality Tests
WHITE PLAINS, NEW YORK — MAY 19, 2009 — ConsumerLab.com reported today that among twelve magnesium supplements selected for testing, only nine met quality standards. One supplement contained only 45.
Recalls & Warnings
January 17, 2018
Joint and Pain Relief Product Contains Undeclared Drug
On January 17, 2018 Health Canada warned consumers that Linsen Double Caulis Plus, a supplement for joint pain, was found to contain the undeclared drug dexamethasone.
Recalls & Warnings
May 30, 2017
Maker of Collagen Supplement Warned for Manufacturing Violations
On July 17, 2014, the FDA issued a warning letter to Morhaim Pharmalab, Inc.
Recalls & Warnings
November 29, 2016
Seller of Mineral, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On July 1, 2016, the FDA issued a warning letter to BioGenyx-Basic Reset, following a facility inspection which found the company's products, including Ionyte, Aqualyte, Beta Factor, Bee Gold and Primo Java to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
June 20, 2013
Glucosamine Legal Settlement Over Labeling -- Many Products Covered
On May 16, 2013, a U.S. District Court preliminarily approved a settlement of class action cases alleging that certain claims made on the labeling of specific glucosamine products are false, deceptive, and misleading. No allegations related to safety or physical injury have been made.
Recalls & Warnings
August 31, 2016
Seller of Joint Supplement Warned for Manufacturing Violations, Drug Claims
On July 15, 2016, the FDA issued a warning letter to Vitalife Inc.
Recalls & Warnings
May 24, 2016
Seller of Joint Health, Omega-3 Supplements & More Warned for Manufacturing Violations, Drug Claims
On May 13, 2016, the FDA issued a warning letter to Rocky Fork Formulas, Inc.
Recalls & Warnings
March 28, 2015
Settlement Restricts Label Claims for Certain Glucosamine Supplements
On March 23, 2015 a federal judge approved a settlement agreement which will require the maker of glucosamine products (Perrigo Co.
Recalls & Warnings
September 14, 2016
Seller of Aloe, Prostate and Joint Supplements Warned for Manufacturing Violations
On April 8, 2016, the FDA issued a warning letter to Salud Natural Entrepreneurs, Inc.
Recalls & Warnings
November 02, 2012
Maker of Joint Health, Blood Sugar, Prostate, Ginkgo Supplements and More Warned For Drug Claims, Misbranding and Adulteration
On September 10, 2012, the FDA issued a warning letter to Global Source Management & Consulting for making drug claims about the following dietary supplements: Scientific Joint Program capsules, Alpha Lipoic Acid, Healthy Blood Sugar Diabetic Support, Dr.
Recalls & Warnings
July 16, 2019
FDA Warns Seller of Magnesium and CBD
On July 9, 2019, the FDA issued a warning letter to Ceba-Tek, Inc.
Clinical Update
7/28/2020
Fish Oil for Arthritis?
Fish oil has shown modest benefits in rheumatoid arthritis, but can it help with osteoarthritis -- inflammation due to worn joints? Find out what a recently published study showed in the What It Does section of the Fish Oil Supplements Review. Also see our Top Picks for fish oil.
Clinical Update
12/15/2018
Pycnogenol for Prostate?
Pycnogenol, a branded form of pine bark extract, is promoted for a variety of uses, including improvement of circulation, cognition, joint pain and vision. It was recently given to men with benign prostatic hyperplasia (BPH) to see if it would improve urinary symptoms. Find out if it worked, and learn if it helps with other conditions, in our updated answer to the question: What is Pycnogenol and does it work? >>
Clinical Update
8/11/2018
CBD for Dogs
Can CBD help dogs with osteoarthritis (worn joints)? Learn what a recent study found (as well as the dose used and a potential side effect) in the CBD for Pets section of the CBD & Hemp Supplements Review. (Also see our Top Picks for CBD for pets – as well as for people).
Clinical Update
5/05/2020
CBD for Osteoarthritis Pain
A recent study, conducted in dogs, showed promise for CBD in reducing pain and inflammation due to osteoarthritis (pain due to worn joints). See the details in the What It Does section of the CBD and Hemp Extracts Review. Also see our Top Picks for CBD for people and for pets.
Clinical Update
6/27/2020
Fish Collagen Allergy
Be aware that some people may be allergic to fish collagen even if they don't respond to common fish allergy tests, according to a recent study. Get the details in the Concerns and Cautions section of the Collagen Supplements Review. Also see our Top Picks for collagen supplements for skin and for joints.
Clinical Update
5/15/2018
Fish Oil for Joints
See what happened when high-dose fish oil was given for a year to older men and women in the Arthritis and Other Inflammatory Diseases section of the Fish Oil Supplements Review. Also see our Top Picks among fish oils and other omega-3 supplements.
Clinical Update
7/16/2019
Has UC-II Collagen Changed?
UC-II is an undenatured collagen that may reduce knee pain. However, one of the more recent studies reported that it contained much less collagen than reported in earlier studies. Has UC-II changed? Find out in our updated answer to the question: What is UC-II and does it help joints?
Clinical Update
10/11/2019
Eggshell Membrane for Bad Knees?
Eggshell membrane, which naturally contains collagen, was tested as a supplement to improve knee pain, stiffness, and function. Find out if it worked in the Joint Pain section of the Collagen Supplements Review. Also see our Top Picks for collagen.
Clinical Update
6/07/2017
Chondroitin Matches Celebrex for Knee Pain
A type of chondroitin was found to reduce the pain of knee osteoarthritis as much as the drug Celebrex, although not as quickly. Due to potential side-effects with Celebrex, the researchers suggested that this form of chondroitin be considered a first-line therapy for knee osteoarthritis. For details, including dosage, see the "What It Does" section of the Joint Supplements Review (Glucosamine, Chondroitin, MSM, and Boswellia), which includes CL's tests and comparisons of products.
Clinical Update
3/08/2016
Knee Arthritis & Vitamin D
People with knee osteoarthritis (worn joints) and generally low vitamin D levels given vitamin D for 2 years showed greater improvement in some, but not all, symptoms compared to those given a placebo. Get the details (as well as our product test results) in the Vitamin D Supplements Review >>
Clinical Update
9/19/2021
For Knee Pain, Try This.
A six-month exercise program was shown to reduce knee pain in 72% of people with osteoarthritis. It’s a completely free program that anyone can do from home. Get the details in the ConsumerTips section of our Joint Supplements Review (although no supplements are involved in the program).
Clinical Update
10/19/2021
Alpha Lipoic Acid for Pain?
Can taking alpha-lipoic acid decrease nerve, muscle or joint pain? See what a new study found in the What It Does section of our Alpha Lipoic Acid Review. Also see our Top Picks for alpha-lipoic acid.
Clinical Update
6/28/2021
Hyaluronic Acid for Wrinkles?
Does supplementing with hyaluronic acid reduce wrinkles or improve skin elasticity in middle-aged men and women? See what a new study found in the Aging Skin and Wrinkles section of our Collagen Supplements Review. Also see our Top Picks for collagen supplements for skin and for joints.
Clinical Update
4/20/2021
Probiotic for Rheumatoid Arthritis?
Can taking a probiotic reduce joint swelling in rheumatoid arthritis? See what a new study found in the What It Does section of our Probiotic Supplements Review.
Clinical Update
5/07/2024
Turmeric for Arthritis?
Does supplementing with turmeric extract (curcumin) help reduce joint pain in people with rheumatoid arthritis? Find out what a recent study showed in the Arthritis section of our Turmeric and Curcumin Supplements and Spices Review, which includes our Top Picks for turmeric and curcumin.
Also see: Which supplements help with arthritis?
Clinical Update
3/31/2022
Doctor's Best Settlement & Refund
Doctor's Best is providing a partial refund to customers of its glucosamine supplements as part of a legal settlement. For details, see the Update to our Joint Supplements Review.
Clinical Update
7/01/2022
Potassium for Arthritis?
Can increasing potassium intake reduce joint pain in people with rheumatoid arthritis? See what a recent study found in the Rheumatoid Arthritis section of our Potassium Supplements Review.
Also see our CL Answer about Supplements for Arthritis.
Clinical Update
6/07/2022
Eggshell Membrane for Osteoarthritis?
Eggshell membrane – a source of collagen – may reduce pain in people with osteoarthritis if taken at a certain dose, according to a recent study. Get the details in the Joint Pain section of our Collagen Supplements Review.
Clinical Update
7/11/2023
Arthritis Supplement May Have Caused Cushing Syndrome
We’ve added more information about the risks of a supplement for joint pain sold by Walmart. Get the details.
Clinical Update
8/01/2023
Mad Cow Disease from Collagen or Gelatin?
Can collagen or gelatin (including gelatin capsules) be contaminated with the infectious agent that causes "mad cow disease"? Find out. Also see our tests of collagen supplements and our Top Picks for skin & wrinkles and Top Picks for joints & tendons.
Clinical Update
12/20/2022
Collagen for Wrinkles?
How much did collagen supplementation reduce the severity of "crow’s feet" wrinkles around the eyes in a recent study? Find out in the Aging skin and wrinkles section of our Collagen Supplements Review. Also see our Top Picks among collagen supplements for skin and for joints.
Recalls & Warnings
December 12, 2018
Seller of Protein Powder, Spirulina & Other Products Warned for Manufacturing Violations
On October 31, 2018, the FDA issued a warning letter to DynaPro International, Inc.
Recalls & Warnings
December 05, 2018
Seller of Cold and Flu Supplements, "SuperFood" & More Warned by FDA
On October 30, 2018, the FDA issued a warning letter to American Botanical Pharmacy following a review of the company's website (www.herbdoc.com) and Facebook page, which found statements made about some of its products to be drug claims.
Recalls & Warnings
December 01, 2018
Seller of 5-HTP, Potassium & More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to The Delano Company, Inc.
Recalls & Warnings
December 19, 2020
FDA Finds Unapproved Drugs in Many Weight Loss and Sexual Enhancement Products Sold Online
On December 18, 2020, the FDA warned consumers that certain products promoted for weight loss, body building, sexual enhancement, pain relief, sleep, and other uses because may contain harmful ingredients.
News Release
April 28, 2009
40% of Green Tea and Selenium Products Fail ConsumerLab.com Review of Cancer-Prevention Supplements; Lycopene Supplements Pass
WHITE PLAINS, NEW YORK — APRIL 28, 2009 — ConsumerLab.com announced results today of its Product Review of Supplements for Cancer Prevention. Among the products that ConsumerLab.com selected for testing, quality problems were found with two out of five green tea supplements.
Recalls & Warnings
November 25, 2017
Seller of "BounceBack" Joint Supplement and Others Products Warned for Drug Claims
On November 14, 2017, the FDA sent a warning letter to Mannatech Incorporated following a facility inspection that found the products BounceBack, MannaBears, ImmunoSTART, TruSHAPE, and Catalyst to be in violation of the Current Good Manufacturing ...
Recalls & Warnings
May 15, 2018
Seller of Apple Cider Vinegar, Joint Supplements & More Warned for Drug Claims
On April 25, 2018, the FDA issued a warning letter to Baker's Best Health Products, Inc.
Recalls & Warnings
August 29, 2017
FDA Warns Seller of Supplements for Allergies, Joint Pain, Bone Health, and More
On August 16, 2017, the FDA issued a warning letter to Total Nutrition, Inc.
News Release
December 09, 2008
Tests of zinc supplements by ConsumerLab.com show only some provide dosage proven to shorten colds, reduce eye disease. Lead contamination found in one product.
WHITE PLAINS, NEW YORK — DECEMBER 9, 2008 — Zinc supplements can shorten colds and reduce the progression of advanced macular degeneration, among other uses. But a new report by ConsumerLab.
Recalls & Warnings
February 25, 2017
Radio "Infomercials" for Cognitive and Joint Health Supplements Deceived Consumers, Says FTC
On February 22, 2017, the Federal Trade Commission and the Maine Office of the Attorney General announced a complaint the marketers of CogniPrin and FlexiPrin, charging that they made misleading claims about the supplements in radio infomercials deceptively formatted as talk shows.
Recalls & Warnings
January 02, 2012
FDA Warns XYMOGEN of Manufacturing and Labeling Violations Regarding Multiple Products
On December 13, 2011, the U.S. FDA sent a Warning Letter to Atlantic Pro Nutrients, Inc. (dba XYMOGEN) regarding labeling and/or manufacturing violations relating to its products, which include Borage CP-240, CoQmax CF, Immune Rx, Iron Glycinate, and Green Tea 600.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
January 21, 2018
Joint Supplement Recalled for Salmonella Risk
On January 21, 2018, Arthri-D, LLC announced a recall of its joint pain supplement, Arthri-D (120 count), due to possible Salmonella contamination.
Clinical Update
1/13/2013
Vitamin D and Knee Arthritis?
Can taking vitamin D reduce the pain and progression of osteoarthritis of the knee? A new study found no benefit for people who already had sufficient levels of vitamin D. In fact, these people needed more anti-inflammatory medication during the study than those given placebo. However, as seen in many other studies of vitamin D, people who entered the study deficient in vitamin D appeared to benefit from being given vitamin D, experiencing reduced pain. For more details, see the update in the Vitamin D Supplements Review, which includes our tests of vitamin D supplements. If you suffer from osteoarthritis of the knee or other joints, also read about glucosamine and chondroitin, as supplements with these ingredients may help. We have tested many of these products.
Recalls & Warnings
March 13, 2018
FDA Warns Seller of Milk Thistle, Chromium, Joint Supplements & More for Manufacturing Violations
On March 9, 2018, the FDA issued a warning letter to Carol Bond Health Foods following a facility inspection which found a number of the company's products, including Dolomite, Lipo Fat Fighter, ThistleX, Injuv, and Vinpocentine, to be ...
Recalls & Warnings
May 12, 2018
Seller of Astaxanthin, Cinnamon, Pine Bark and More Warned for Manufacturing Violations
On March 22, 2018, the FDA issued a warning letter to Get The Tea following a facility inspection which found the company's products, including Get the Tea Astaxanthin Max, Get the Tea Colostrum Complete, and Get the Tea Pine Bark Capsules, to be adulterated because they were ...
Recalls & Warnings
October 18, 2017
Total Body Nutrition Warned for Banned Stimulant in Supplements
On September 28, 2017, the FDA issued a warning letter to Total Body Nutrition following a facility inspection which found some of the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
September 23, 2014
Maker of Joint and Weight Products Warned for Hidden Drugs, Manufacturing Violations
On September 15, 2014, the FDA issued a warning letter to West Coast Laboratories, Inc., because the company's joint health supplements, Super ArthGold and Pro ArthMax, were found to contain hidden drugs.
Recalls & Warnings
May 10, 2013
Protein Extract Recalled for Undeclared Allergens
On May 6, 2013, Pure Herbs Ltd. issued a voluntary recall of Protein Extract because it contains undeclared milk and soy allergens.
Recalls & Warnings
September 10, 2014
Seller of Joint Pain Supplement Warned for Drug Claims
On August 24, 2014, the FDA issued a warning letter to CreAgri, Inc., following a website review, which found statements made about the company's products, including Olivenol plus Easeflex, Olivenol plus Essence Capsules, and Olivenol plus Essence Elixer, to be drug claims.
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
February 28, 2013
Maker of Liquid Omega-3 Supplements Warned For Manufacturing Violations
On February 20, 2013, the FDA issued a warning letter to Capco Custom Packaging Inc.
Recalls & Warnings
February 13, 2013
Herbal Supplement Maker Warned For Manufacturing Violations, Misbranding
On February 6, 2013, the FDA issued a warning letter to Genesis Herb Company LLC, following a facility inspection which found the company's Seizure, Relax, Formula ARTH, Disintegrate, Gout, Plantain, and Colloidal Silver dietary supplements to be adulterated because they were prepared, packed, or ...
Recalls & Warnings
October 25, 2016
Seller of Liquid Aloe & Mineral Supplement Warned for Manufacturing Violations
On August 10, 2016, the FDA issued a warning letter to Perfect Source Natural Products Inc.
News Release
November 18, 2008
Adulteration Suspected with Some "Memory" Supplements -- Few Ginkgo and Huperzine Supplements Pass ConsumerLab.com Tests; Quality High for Acetyl-L-Carnitine
WHITE PLAINS, NEW YORK — NOVEMBER 18, 2008 — Tests by ConsumerLab.com of Ginkgo biloba supplements show that few products meet quality standards.
Recalls & Warnings
August 06, 2014
Seller of Mineral and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to Mezotrace Corporation following a facility inspection which found the company's products, including Calcium/Magnesium Natural Minerals & Trace Elements, Calcium/Magnesium Natural Minerals & Trace Elements with Vitamin D, and Calcium/Magnesium ...
Recalls & Warnings
May 02, 2015
Maker of Garcinia, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On March 25, 2015, the FDA issued a warning letter to JW Nutritional LLC, following a facility inspection which found the company's products, including Vanish V2 Domestic, Mr.
Recalls & Warnings
August 21, 2014
Seller of Joint Supplement Warned for Manufacturing Violations and Drug Claims
On July 17, 2014, the FDA issued a warning letter to Klein Laboratories, Inc.
Recalls & Warnings
September 17, 2013
Seller of Omega-3 and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On August 29, 2013, the FDA issued a warning letter to Y.S. Health Corp.
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
June 06, 2015
Joint Supplement Contains Drugs, FDA Warns
On June 5, 2015 the FDA warned consumers not to buy or use joint pain supplement Pyrola Advanced Joint Formula because it was found to contain diclofenac and chlorpheniramine.
Recalls & Warnings
July 02, 2015
Maker of Arthritis Supplement Warned for Manufacturing Violations, Drug Claims
On June 17, 2015, the FDA issued a warning letter to Desert Stream, Inc.
Recalls & Warnings
May 24, 2013
Seller of Liquid Minerals, Joint Care and Herbal Supplements Warned For Manufacturing Violations, Drug Claims
On April 8, 2013, the FDA issued a warning letter to Body Systems, Inc.
Recalls & Warnings
February 27, 2018
Dog Treats, Food Recalled Due to Salmonella, Listeria Risk
The following dog treats and dog food are being recalled because they have the potential to be contaminated with Listeria monocytogenes or Salmonella, which can cause illness in pets who consume these products, as well as in pet owners who handle the contaminated products or touch ...
Recalls & Warnings
February 28, 2013
Maker of Joint, Mood and B Vitamin Supplements Warned For Manufacturing Violations, Misbranding and Drug Claims
On February 11, 2013, the FDA issued a warning letter to Kreativ Health, Inc.
Recalls & Warnings
April 01, 2017
Maker of Prelief, Urinozinc Prostate Health Formula Warned for Manufacturing Violations
On March 16, 2017 the FDA issued a warning letter to DSE Healthcare Solutions, LLC, following a facility inspection which found the company's products, including Prelief and Urinozinc Prostate Health Formula to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
December 05, 2014
Maker of Ginseng, Zinc, Calcium and More Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Long Island Pharmaceuticals, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
May 02, 2017
Seller of Amino Acid Supplements & More Warned for Manufacturing Violations
On April 20, 2017 the FDA issued a warning letter to Naturecom Inc.
Recalls & Warnings
April 04, 2017
Seller of B Vitamins, Vitamin C, Potassium & More Warned for Manufacturing Violations
On March 24, 2017 the FDA issued a warning letter to The Sanapac Company, Inc.
Recalls & Warnings
May 16, 2015
FTC Mails Refund Checks to Nopalea Consumers
On May 15, 2015, the FTC announced it is mailing almost 500,000 checks totaling approximately $3 million to consumers who purchased the cactus-based fruit drink Nopalea (TriVita Inc).
Recalls & Warnings
February 20, 2013
FDA Warns USPLabs For Adulteration and Drug Claims
On December 4, 2012 the FDA issued a warning letter to USPLabs, LLC following facility inspections which found the company's products, including dietary supplements Jacked3d, OxyElite Pro, Prime, and Super Cissus, to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
April 24, 2014
Arthritis Supplement Containing Multiple Drugs Recalled
On April 21, 2014, Nano Well-being Health Inc., issued a voluntary recall of two lots of arthritis supplement Super Arthgold, because it was found to contain the drugs chlorzoxazone, diclofenac and indomethacin.
Recalls & Warnings
November 20, 2020
FTC Files Complaint Against Two Supplement Companies for Deceptive Marketing
On November 20, 2020, the FTC approved a Part 3 administrative complaint against Health Research Laboratories, LLC, its owner Kramer Duhon, and Whole Body Supplements, LLC for making unverified claims that their products can prevent or treat diseases.
Recalls & Warnings
February 13, 2014
FDA Warns Consumers Not To Buy or Use Arthritis Supplement
On February 13, 2014, the FDA warned consumers not to buy or use arthritis supplement Arth-Q because it was found to contain ibuprofen.
Recalls & Warnings
December 02, 2014
Glucosamine Class-Action Settlement Reversed by Appeals Court
On November 19, 2014, an appeals court rejected a settlement of a class-action lawsuit that alleged label claims on glucosamine products manufactured or sold by Rexall Sundown, Inc., NBTY, Inc. or their affiliates, were false, deceptive or misleading.
Recalls & Warnings
January 21, 2017
Seller of "Cancer Kits," Alpha Lipoic Acid Supplements and More Warned for Drug Claims
On December 22, 2016, the FDA issued a warning letter to Northern Health Products, Inc. following a review of the company's websites, www.northernhealthproducts.com and www.petdca.
Recalls & Warnings
April 24, 2023
FTC Warns Almost 700 Companies: Unsupported Health Claims May Result in Substantial Civil Penalties
On April 13, 2023, the Federal Trade Commission (FTC) notified approximately 670 companies that market over-the-counter (OTC) drugs, homeopathic products, dietary supplements, or functional foods that they could incur significant civil penalties if they make unsubstantiated claims about the ...
Recalls & Warnings
March 01, 2013
Manufacturer of Joint, Weight Loss, Muscle Supplements and More Warned for Adulteration, Misbranding and Drug Claims
On February 4, 2013, the FDA issued a warning letter to DC Nutrition, Inc.
Recalls & Warnings
September 27, 2016
Seller of B Vitamins, Omega-3s and More Warned for Manufacturing Violations, Drug Claims
On September 15, 2016, the FDA issued a warning letter to Positive Power Nutrition, following a facility inspection which found the company's products, including High Energy C-Complex, Positive Vitality, Positive Performance, Positive CardioGuard, B-Complex 100, Positive Essentials, Positive ...
Recalls & Warnings
February 14, 2017
Life Extension Warned for Drug Claims
On February 1, 2017, the FDA issued a warning letter to Life Extension Foundation Buyers Club, Inc.
Recalls & Warnings
March 29, 2016
Maker of Bone Health, Prostate, Cholesterol Supplements and More Warned for Drug Claims
On March 17, 2016, the FDA issued a warning letter to Rx Vitamins, Inc., following a facility inspection which found the company's products, includingTestost-Rx, DB-7, The Bone Density Formula, NaturLo Cholesterol, ThyRx-7 and Arth-9 to be misbranded.
Recalls & Warnings
June 07, 2017
FDA Warns Seller of Probiotic and Omega-3 Supplements for Manufacturing Violations
On May 22, 2017, the FDA issued a warning letter to BioTE Medical, LLC following a facility inspection which found its products, including BioTE DIM, BiotTE Probiotic, BioTE Iodine Plus, and BioTE Omega 3 to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
March 04, 2022
FTC, FDA, and DOJ Take Joint Action Against Herbal Tea Companies for COVID Claims
On March 3, 2022, the FDA, FTC, and DOJ sued a New York-based marketer of herbal tea, in attempts to permanently block deceptive ads that claim Earth Tea is clinically proven to treat, cure, and prevent COVID-19.
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
February 07, 2017
Seller of Chinese Herbal Products for Prostate, Menopause and More Warned for Manufacturing Violations
On January 23, 2017, the FDA issued a warning letter to Herbal Sciences International, Inc.
Recalls & Warnings
January 17, 2017
Seller of Heart, Senior and Teen Supplements Warned For Drug Claims
On December 7, 2016, the FDA issued a warning letter to Esteem Products Ltd following a review of the company's website, which found statements made about its products, Cardio Life, Total Man, Total Woman, Senior Total Man, Senior Total Woman , and Total Teen to be drug ...
Recalls & Warnings
January 07, 2017
Seller of Protein and Workout Supplements Warned for Manufacturing Violations, Label Errors
On December 22, 2016, the FDA issued a warning letter to Rock Solid Nutrition, LLC, following a facility inspection which found the company's products, including Whey Isolate (Cinnabun flavor), Pre-Pump (Massive Mango flavor) and Strength Test to be adulterated because ...
Recalls & Warnings
October 08, 2016
Seller of Cell Power and Super Silica Warned for Manufacturing Violations, Misbranding
On September 23, 2016, the FDA issued a warning letter to SSO, Inc.
Recalls & Warnings
September 06, 2016
Seller on Noni Juice Warned for Manufacturing Violations, Drug Claims
On August 26, 2016, the FDA issued a warning letter to Healing Noni Co. L.L.C.
Recalls & Warnings
August 23, 2016
Seller of Aloe Liquid and Capsules Warned for Manufacturing Violations, Drug Claims
On July 15, 2016, the FDA issued a warning letter to Aloe Farms, Inc.
Recalls & Warnings
August 02, 2016
Seller of Whey Protein Warned for Manufacturing Violations, Drug Claims
On July 22, 2016, the FDA issued a warning letter to New Horizon Nutraceuticals, LLC, following a facility inspection which found the company's product, One World Whey Protein Power Food to be adulterated because it was prepared, packed, or held under conditions that violate Current ...
Recalls & Warnings
July 23, 2016
Maker of "Super Food" Warned for Manufacturing Violations
On July 12, 2016, the FDA issued a warning letter to TerraVare, Inc.
Recalls & Warnings
November 21, 2014
Herbal Arthritis Supplement Found to Contain Undeclared Drugs
On November 20, 2014, the FDA warned consumers not to purchase or use the herbal dietary supplement Feng Shi Ling because it was found to contain the undeclared drugs diclofenac and indomethacin.
Recalls & Warnings
February 21, 2013
Pet Supplement Company Issues Recall Due To Potential Bacterial Contamination
On February 20, 2013, animal supplement company Nutri-Vet, LLC issued a recall of Nutri-Vet and NutriPet Chicken Jerky Products because they may be contaminated with Salmonella.
Recalls & Warnings
May 17, 2013
Maker of Cardio, Arthritis, Cleanse Supplements and More Warned For Manufacturing Violations and Drug Claims
On May 8, 2013, the FDA issued a warning letter to Entrenet Nutritionals, Inc.
Recalls & Warnings
May 06, 2015
Joint Pain Supplements Found to Contain Diuretics, Antihistamines and Other Undeclared Drugs
On May 4, 2015, the FDA warned consumers not to buy or use the following supplements promoted for joint or back pain because they were found to contain undeclared drugs. Each was identified during an examination of international mail shipments.
Recalls & Warnings
March 01, 2023
Seller of Dr. Miller’s Herbal “Detox” Teas Warned by FDA for Drug Claims
On February 3, 2023, the FDA issued a warning letter to Jackson Health & Wellness Clinic after inspection of the company’s website found statements about the company’s Dr. Miller’s Holy Tea, Dr. Miller’s Super Holy Tea, Dr. Miller’s Ultimate Tea, Dr.
Recalls & Warnings
December 01, 2022
FTC Takes Action Against Company Promoting “COVID Resist” Supplement to Treat COVID-19
On November 22, 2022, the FTC filed a complaint in a U.S. district court against California-based company Precision Patient Outcomes, Inc. for promoting its COVID Resist and VIRUS Resist supplements to prevent and treat COVID-19.
Recalls & Warnings
November 06, 2023
FDA Warns Consumers Not to Use Dr. Ergin’s SugarMD Advanced Glucose Support, Found to Contain Prescription Drugs
On November 3, 2023, the FDA warned consumers not to purchase or use Dr. Ergin’s SugarMD Advanced Glucose Support products after FDA laboratory analysis found the supplement to contain glyburide and metformin.
News Release
April 24, 2007
ConsumerLab.com reports improvement in quality of SAM-e supplements used for osteoarthritis and depression — Recent testing shows quality is up since studies in 2000 and 2003
WHITE PLAINS, NEW YORK — April 24, 2007 — ConsumerLab.com announced that all eight SAMe supplements that it recently selected for review passed quality testing — an improvement over tests in previous years. SAMe (S-adenosyl-methionine) is used to treat depression and joint pain.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
September 24, 2020
FTC Sends Refund Checks to Consumers of Deceptive Joint Pain Supplement
On September 24, 2020, the FTC announced it is mailing 4,782 checks totaling more than $76,000 to consumers who bought Isoprex, a supplement marketed with deceptive claims that it could treat or cure joint pain, muscle pain, headaches, and arthritis.
Recalls & Warnings
February 02, 2021
Seller of Aloe Products Warned for Claiming to Treat Joint Stiffness
On January 22, 2021, the FDA issued a warning letter to American Global Health Group, LLC following a review of the company's website, which found statements made about the company's products AloeCure VeraFlex, AloeCure Advanced Formula Capsule, AloeCure Pure Aloe Vera ...
News Release
April 11, 2007
Many arthritis supplements lack key listed ingredient — ConsumerLab.com finds chondroitin missing or low in eight supplements; Glucosamine and MSM also tested
WHITE PLAINS, NEW YORK — April 11, 2007 — Forty percent of the supplements for osteoarthritis that ConsumerLab.com recently selected for testing were found to lack all or some of their key ingredients. Osteoarthritis is the most common type of arthritis, affecting an estimated 20.
Recalls & Warnings
November 06, 2014
Seller of Cholesterol, Arthritis Supplements and More Warned for Drug Claims
On October 17, 2014, the FDA issued a warning letter to Vitamins Direct (USA) and Golden Pride, Inc., which distribute Flexezy and Physician's Signature brands of dietary supplements, following a facility inspection which found statements made about certain supplements to be drug claims.
Recalls & Warnings
December 21, 2016
Seller of 5-HTP, Chromium, Curcumin and More Warned for Drug Claims
On December 1, 2016, the FDA issued a warning letter to Aurora Health and Nutrition following a review of the company's website, which found statements made about its products, 5-HTP, Alpha Lipoic Acid, the Argentyn 23 product line, Artecin 90 Vegetarian Capsules, ...
Recalls & Warnings
April 17, 2020
Joint Pain Supplement Isoprex Settles Charges of Making False Claims
On April 16, 2020, Renaissance Health Publishing, LLC, agreed to halt their allegedly deceptive advertising claims about their Isoprex supplement that targeted older consumers nationwide after the Federal Trade Commission (FTC) filed a complaint.
Recalls & Warnings
June 09, 2020
Six More Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On June 5, 2020, the FTC announced that it sent warning letters to six multi-level marketing companies for selling products such as immune system boosters and probiotics with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrepresenting potential earnings people who have ...
News Release
March 06, 2007
ConsumerLab.com produces series of free online seminars about dietary supplements — First seminar on "joint health" now available
WHITE PLAINS, NEW YORK — MARCH 6, 2007 — ConsumerLab.com announced today that it is producing and webcasting a series of free, online audio seminars, or "webinars," that explain the use of popular types of dietary supplements.
Recalls & Warnings
November 28, 2014
Seller of Herbal Extracts Warned for Manufacturing Violations, Drug Claims
On November 3, 2014, the FDA issued a warning letter to Avena Botanicals, Inc.
News Release
February 14, 2007
ConsumerLab.com reports on anti-depressant herb — St. John's Wort — Few supplements pass quality testing
WHITE PLAINS, NEW YORK — FEBRUARY 14, 2007 — St. John's wort may be helpful in treating mild to moderate cases of clinical depression, but a new report from ConsumerLab.com shows that few of the herbal products tested met quality standards. Americans purchased $64 million of St.
News Release
December 12, 2006
Probiotic supplements grow in popularity but viable bacteria missing in many — ConsumerLab.com cautions consumers to select probiotics carefully and store them properly
WHITE PLAINS, NEW YORK — DECEMBER 12, 2006 — Sales of supplements containing beneficial organisms (probiotics) have grown rapidly, but a new test report from ConsumerLab.com shows that 44% contained fewer viable organisms than claimed or generally known to be effective.
News Release
November 13, 2006
Tests of "muscle" supplements finds some "weak" products but most contain expected creatine, HMB, or amino acids — Review of muscular enhancement supplements published by ConsumerLab.com
WESTCHESTER, NEW YORK — MONDAY, NOVEMBER 13, 2006 — Bodybuilders and athletes often turn to supplements to enhance muscle size and strength.
Recalls & Warnings
May 14, 2014
Maker of Mood, Smoking Cessation Supplements and More Warned for Violations, Drug Claims
On April 17, 2014, the FDA issued a warning letter to CDJ Holding, Incorporated, d.b.a.
Recalls & Warnings
June 16, 2020
FDA Warns Four Companies for Unsafe "Homeopathic" Injectables
On June 16, 2020, the FDA issued warning letters to four manufacturers of unapproved injectable drugs labeled as homeopathic.
Recalls & Warnings
April 30, 2021
FDA Warns Seller of Fish Oil, Apple Cider Vinegar, Collagen, and Elderberry Supplements
On April 13, 2021, the FDA issued a warning letter to Orphic Nutrition following a review of the company's websites, which found statements made about the products Apple Cider Vinegar Capsules, Ashwagandha Capsules, Garcinia Cambogia Capsules, Premium Collagen Peptides Capsules, Elderberry ...
Recalls & Warnings
April 10, 2021
Companies Deceived Consumers About Fish Oil for Liver Disease, Says FTC
On April 1, 2021, the Federal Trade Commission (FTC) announced that fish oil supplement manufacturer BASF SE and its U.S.
Recalls & Warnings
June 22, 2021
Living Free Vitamins, Joint, Nerve and Other Supplements Recalled
On June 21, 2021, Bea Lydecker's Naturals, Inc. issued a recall of six Living Free brand supplements because the labels do not declare soy lecithin.
Recalls & Warnings
May 30, 2020
FTC Sends Refund Checks for TrueAloe and AloeCran
On May 26, 2020, the FTC announced it is mailing 22,581 checks totaling more than $470,000 to consumers who purchased deceptively marketed supplements, TrueAloe and AloeCran.
News Release
October 20, 2006
ConsumerLab.com report on CoQ10 supplements finds quality high but large range in suggested dosage
WHITE PLAINS, NEW YORK — OCTOBER 20, 2006 — New test results from ConsumerLab.com for CoQ10 supplements show that all of the products selected contained amounts of ingredient consistent with their labels.
News Release
November 18, 2003
ConsumerLab.com reports improvement in quality of SAM-e supplements used for osteoarthritis and depression — But a high priced product found to contain only 30% of label claim
WHITE PLAINS, NY — November 18, 2003 — ConsumerLab.
News Release
September 24, 2003
Consumerlab.com finds new forms of creatine prone to problems — Test results of muscular enhancement supplements published online today
WHITE PLAINS, NY — September 24, 2003 — Testing by ConsumerLab.com of creatine supplements found problems with the majority of products sold in liquid, effervescent and chewable forms. Problems were not found among standard creatine "powder" products.
News Release
August 28, 2003
Some problems persist with ginseng supplements but overall quality improves according to ConsumerLab.com — Test results published online today
WHITE PLAINS, NY — August 28, 2003 — In contrast to its testing three years ago in which nearly 60% of Asian and American Ginseng supplements were found to have significant problems, testing of recently purchased products by ConsumerLab.
News Release
July 31, 2003
ConsumerLab.com finds many cholesterol-lowering supplements poorly made — Test results published online today
WHITE PLAINS, NY — July 31, 2003 — ConsumerLab.com reported today that more than half of the cholesterol-lowering supplements that it recently purchased failed to contain their listed ingredients and/or could not adequately break apart to release their contents.
News Release
July 10, 2002
Pharmavite dietary supplements receive ConsumerLab.com approval — Vitamin E, SAM-e, St. John's Wort, Ginkgo and others merit approved quality products ranking
WHITE PLAINS, NY — July 10, 2002 — ConsumerLab.
Recalls & Warnings
April 28, 2020
Natures CBD Oil Distribution Warned for Joint Pain Claims
On April 20, 2020, the FDA issued a warning letter to Homero Corp DBA Natures CBD Oil Distribution, following an inspection of the company's website which found the company's products, including Natures Pure CBD Oil, CBD Froggies 25mg, CBD Froggies 50mg, CBD Edibles 200mg Frogs, CBD ...
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
July 10, 2021
FDA Warns Seller of Omega XL
On June 31, 2021 the FDA issued a warning letter to Great Healthworks, Inc. following a review of the company's website, which found statements made about Omega XL and ProbioticXL supplements to be drug claims.
Recalls & Warnings
May 10, 2021
Collagen & Biotin Supplement Recalled Due to Allergen Risk
On May 6, 2021, Bloommy, Inc. issued a recall of Bloommy Biotin Collagen Keratin Capsules for Skin, Joint, and Hair due to the presence of a fish allergen for the collagen-based ingredient.
Recalls & Warnings
August 17, 2021
Love & Peas Protein Rich Meal Replacement Recalled
On July 30, 2021, Nature's Sunshine issued a recall of certain lots of Love & Peas Protein Rich Meal Replacement because it may contain milk.
Recalls & Warnings
October 13, 2018
Pharmaceutical Drugs Found In Dietary Supplements Pose Danger to Consumers
Almost 800 dietary supplements sold between 2007 and 2016 contained unapproved pharmaceutical ingredients, according to a study published today in the Journal of the American Medical Association (JAMA).
Recalls & Warnings
March 18, 2021
Don't Drink Real Water Alkaline Water, FDA Warns After Reports of Liver Illness
On March 16, 2021, the FDA warned consumers not to drink or use Real Water bottled alkaline water while it investigates reports of hepatitis associated with the product.
Recalls & Warnings
August 20, 2019
Seller of Liposomal Curcumin, Vitamin C & Melatonin Warned for Manufacturing Violations
On August 6, 2019, the FDA issued a warning letter to Let's Talk Health, Inc.
Recalls & Warnings
November 27, 2018
More Dog Food Recalled Due to Risk of Vitamin D Toxicity
On November 27, 2018, Sunshine Mills, Inc. issued a recall of select Evolve Puppy, Sportsman's Pride Large Breed Puppy and Triumph Chicken and Rice Dog Food because they may contain elevated levels of vitamin D.
Recalls & Warnings
November 06, 2018
Dog Food Recalled After Reports of Vitamin D Toxicity
On November 2, 2018, Natural Life Pet Products and Nutrisca issued a recall of certain dry dog foods because they contain elevated levels of vitamin D. The elevated levels of vitamin D were found during an investigation into several reports of vitamin D toxicity by pet owners.
Recalls & Warnings
August 09, 2002
Recall of 7 Herbal Products from Botanic Lab in US and Abroad
The U.S. Food and Drug Administration (FDA) released the following Class II recall information in its August 7, 2002 Enforcement Report.
Recalls & Warnings
November 06, 2014
Seller of Prostate, Heart Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Health Research Laboratories, LLC/New World Health, following a review of the company's websites, which found statements made about AtheChel Advanced, Betarol, BioTherapex, Omega-3 Cardio Plus, RejuvaLifeRx, and Ultimate Health Formula to be ...
Recalls & Warnings
September 26, 2014
FDA Warns Seller of Supplements and Chocolate Promoted to Treat Ebola
On September 23, 2014, the FDA issued a warning letter to Natural Solutions Foundation, following a review of the company's websites which found statements made about Silver Sol Nano Silver (also called The Silver Solution) and CBD Organic Dark Chocolate Bars (also called High Potency CBD Hemp Oil) ...
Recalls & Warnings
October 10, 2014
Seller of Multivitamin Warned for Drug Claims
On September 25, 2014, the FDA issued a warning letter to Multimmunity, Inc., following a review of the company's website which found statements made about the dietary supplement Multimmunity to be drug claims.
Recalls & Warnings
December 01, 2017
Health Research Labs Agrees to Settle FTC Charges of False Claims, Deceptive Marketing of BioTherapex and NeuroPlus
On November 30, 2017, the FTC announced Health Research Laboratories LLC and owner, Kramer Duhon, agreed to settle charges that they made false claims about the company's products, BioTherapex and NeuroPlus.
Recalls & Warnings
June 21, 2002
Canadian Warning on Use of Seven Herbal Supplements from BotanicLab
On June 19, 2002, Health Canada issued a warning that Canadians not use seven herbal products manufactured in the United States by BotanicLab because they contain undeclared prescription drugs that could cause serious health effects if not taken under medical supervision.
Recalls & Warnings
September 26, 2014
Sellers of Essential Oils Warned for Claiming to Treat Ebola, Other Diseases
On September 22, 2014, the FDA issued a warning to Young Living following a review of websites, Pinterest, Facebook and Twitter accounts owned by Young Living distributors, which found statements made about the company's essential oils, including Thieves, Cinnamon Bark, Oregano, ImmuPower, ...
Recalls & Warnings
September 26, 2014
Seller Warned for Promoting Essential Oils to Treat Flu, MRSA, Measles and More
On September 22, 2014, the FDA issued a warning to doTERRA International, LLC.
Recalls & Warnings
June 13, 2012
Tart Cherry Supplement Selller Warned by FDA Over Illegal Health Claims
The U.S. FDA recently published a Warning Letter (dated June 8, 2012) to Michelle's Miracle, Inc. for illegally promoting a range of tart cherry dietary supplements using health claims reserved for drugs. The products cited are:
Recalls & Warnings
May 03, 2011
FDA Warns: Beware of Bogus STD Products
On May 3, 2011, the U.S. Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) announced a joint effort to remove products from the market that make unproven claims to treat, cure, and prevent sexually transmitted diseases (STDs).
Recalls & Warnings
March 28, 2008
FDA Warns of Adverse Reactions with Two Liquid Supplements -- Selenium Toxicity Possible
The U.S. Food and Drug Administration is advising consumers not to purchase or consume Total Body Formula in the flavors of Tropical Orange and Peach Nectar, or Total Body Mega Formula in the Orange/Tangerine flavor.
Recalls & Warnings
August 26, 2014
Seller of Immune Supplement Warned for Drug Claims
On August 12, 2014, the FDA issued a warning letter to Ad-Med Biotechnology, following a review of the company's websites and product labels, which found statements made about Immune Active Adult Formula and Immune Active Children's Formula to be drug claims.
Recalls & Warnings
September 26, 2015
Seller of Muscle Supplements and More Warned for Manufacturing Violations
On August 14, 2015, the FDA issued a warning letter to Chaotic Labz, Inc.
Recalls & Warnings
September 05, 2015
Maker of Multivitamin and Fish Oil Warned for Manufacturing Violations
On July 22, 2015, the FDA issued a warning letter to Westar Nutritional Corp. dba Viva Life Science, Inc.
Recalls & Warnings
August 26, 2014
Supplement Maker Warned for Manufacturing Violations
On August 14, 2014, the FDA issued a warning letter to dietary supplement manufacturer Big Easy Confections following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing ...
Recalls & Warnings
April 11, 2008
Twelve Dietary Herbal Supplements Recalled -- Possible Health Risk Associated with Ephedra, Aristolochic Acid and Human Placenta
On April 10, 2008, the FDA posted a recall notice issued the same day from Herbal Science International, Inc. (AKA Jen-On Herbal Science International, Inc.).
Recalls & Warnings
January 21, 2015
Supplement Maker Ordered to Stop Selling Products
On January 15, 2015, a federal judge ordered a permanent injunction against dietary supplement manufacturer Health One Pharmaceuticals, Inc. which requires the company to stop manufacturing and selling dietary supplements.
Recalls & Warnings
September 05, 2014
Seller of "Nerve", "Cleanse" and Probiotic Supplements Warned for Drug Claims
On July 30, 2014, the FDA issued a warning letter to Plexus Worldwide, Inc., following a review of the company's website and product labels, which found statements made about Fast Relief, BioCleanse and ProBio5 to be drug claims.
Recalls & Warnings
October 11, 2016
Seller of Glucosamine and Chondroitin Settles FTC Charges of False Advertising
On October 5, 2016, the FTC announced the sellers of the liquid glucosamine and chondroitin supplement Supple (Supple LLC) agreed to settle charges they made false claims about the product.
Recalls & Warnings
February 05, 2019
Hill's Science Diet, Prescription Diet Dog Food Recalled Due to Risk of Vitamin D Toxicity
Recall Expanded (3/20/19): This recall was expanded to include additional products.
Recalls & Warnings
July 26, 2014
Marketers of Nopal Cactus Drink Settle FTC Charges of Deceptive Claims
TriVita Inc., and marketers of the company's "prickly pear" fruit drink Nopalea have agreed to pay $3.5 million in consumer refunds to settle FTC charges they made deceptive claims that the drink treats health problems ranging from skin conditions to joint pain and respiratory problems.
Recalls & Warnings
October 31, 2014
Seller of "Cleanse," Allergy Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 15, 2014, the FDA issued a warning letter to Evangelical Christian Ministries, dba Health & Herbs, following a facility inspection which found the company's products, CORYDALIS and CVF BGONE to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
May 30, 2015
Seller of Supplements for Herpes, Prostate Cancer & More Warned for Drug Claims
On May 7, 2015, the FDA issued a warning letter to Strictly Health Corporation, following a review of the company's websites which found statements made about FENVIR, Prosta Pep and Tonalin brand CLA to be drug claims.
Recalls & Warnings
January 31, 2015
Maker of Soy and Zinc Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to PreMark Health Science, Inc.
Recalls & Warnings
March 10, 2015
Seller of Vision Supplements Warned for Manufacturing Violations, Drug Claims
On February 26, 2015, the FDA issued a warning letter to Biosyntrx, following a facility inspection which found the company's products, including BioTears Oral Gel Caps, ZoOmega-3 Concentrated Pharmaceutical-Grade Fish Oil, EpiCor A Nutrient-dense High Metabolite Immunogen, Sight C+ Mineral ...
Recalls & Warnings
December 29, 2015
Maker of Vitamin K, Vitamin A & More Warned for Manufacturing Violations, Drug Claims
On December 10 2015, the FDA issued a warning letter to Dherbs Health Emporium, Inc.
Recalls & Warnings
November 18, 2003
Supplement Promoted As Cancer Treatment Removed from Market
On November 10, 2003, the Food and Drug Administration (FDA) announced that NBTY, Inc., of Bohemia, N.Y.
Recalls & Warnings
September 10, 2012
Maker of Omega-3, Bone and Brain and Supplements Warned For Drug Claims
On August 27, 2012, the FDA issued a warning letter to PruTect Rx indicating that statements on the company's websites about Omega3PruTect, NeuroPruTect, VitaminD3PruTect and OsteoPruTect constitute drug claims, although the products are not approved drugs.
Recalls & Warnings
June 04, 2012
FDA Warning on Supplement for Pain Relief
On June 1, 2012, the U.S. FDA warned consumers that Reumofan Plus, marketed as a “natural” dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
Recalls & Warnings
November 18, 2015
Seller of "Hangover" Supplement Warned for Manufacturing Violations & Drug Claims
On September 17, 2015, the FDA issued a warning letter to Life Support Development Ltd, following a facility inspection which found the company's product, including Life Support Hangover Relief to be adulterated because they prepared, packed, or held under conditions that violate ...
Recalls & Warnings
August 23, 2015
Maker of Herbal Supplements Sold on eBay, Amazon and Other Websites Shut Down
On August 17, 2015, a federal court ordered a permanent injunction against dietary supplement company Iowa Select Herbs LLC for unlawfully manufacturing and distributing unapproved new drugs and misbranded drugs and adulterated and misbranded dietary supplements.
Recalls & Warnings
August 11, 2015
Company Ordered to Stop Making and Selling Supplements
On August 4, 2015, a federal court ordered a permanent injunction against dietary supplement company Atrium Inc., and two companies under the same ownerhship, Aspen Group Inc., Nutri-Pak of Wisconsin Inc.
Recalls & Warnings
June 03, 2015
Maker of B-12 Energy Supplement Warned for Manufacturing Violations
On May 15, 2015, the FDA issued a warning letter to LiquidCapsule Manufacturing, LLC.
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
February 24, 2015
Seller of Heart, Brain and Diabetes Supplements Warned for Drug Claims
On February 6, 2015, the FDA issued a warning letter to LCW, Inc.
Recalls & Warnings
January 27, 2015
Seller of Aloe, Sexual Enhancement Supplements and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Aloe Man, Inc., following a facility inspection which found the company's products, including The Aloe Man's Super Bright, Dr. Johnson's Maximum Desire, Dr. Johnson's Body Healer, and Dr.
Recalls & Warnings
January 23, 2015
Maker of Vitamin Drinks Warned for Manufacturing Violations
On January 7, 2015, the FDA issued a warning letter to NYSW Beverage Brands, Inc.
Recalls & Warnings
February 10, 2015
Seller of "Natural" Cough Syrup Warned for Manufacturing Violations, Drug Claims
On January 21, 2015, the FDA issued a warning letter to Fragrance Manufacturing Incorporated, following a facility inspection which found the company's products, including Maty's All Natural Cough Syrup and Maty's All Natural Cough Syrup for Kids, to be adulterated because they ...
Recalls & Warnings
May 23, 2015
Seller of Magnesium, Calcium, Silver and More Warned for Manufacturing Violations, Drug Claims
On May 8, 2015, the FDA issued a warning letter to Pick and Pay, Inc.
Recalls & Warnings
October 30, 2014
Supplement Maker Warned for Manufacturing Violations
On October 21, 2014, the FDA issued a warning letter to DNG Trading & Milling, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
November 11, 2014
Maker of Children's Vitamins, Weight Loss Supplements and More Warned for Manufacturing Violations
On October 30, 2014, the FDA issued a warning letter to VitalHealth Tech, Inc., following a facility inspection which found the company's products, including Kids Mighty Vites tablets, Omni Jr.
Recalls & Warnings
November 11, 2014
Seller of Sexual, Muscle Enhancement Supplements Warned for Manufacturing Violations and Drug Claims
On August 26, 2014, the FDA issued a warning letter to GE Pharma LLC following a facility inspection which found the company's products, including Fire Burn, Fire Storm, Creatine, Amino Fire, Nitric Fire, Jet Fire, Oxy Fire, HGH, Cissus, Hydro shield, Performa-Test, Raspberry Ketones, Fire Drol, ...
Recalls & Warnings
January 17, 2015
Supplement Maker Warned for Manufacturing Violations
On August 5, 2014, the FDA issued a warning letter to Engineering Nutrition, following a facility inspection which found the company's products, which were not named, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for ...
Recalls & Warnings
January 17, 2015
Maker of Magnesium and Potassium Supplements Warned for Manufacturing Violations
On December 23, 2014, the FDA issued a warning letter to Nutri Spec, Inc.
Recalls & Warnings
December 30, 2014
Maker of Probiotic Supplement Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to Wellmill LLC, dba Vitamix, following a facility inspection which found the company's products, including Butcher's Broom powder and Lactobacillus acidophilus powder (used as ingredients in a finished product which was not named) to be ...
Recalls & Warnings
December 30, 2014
Maker of Aloe, Weight Loss Supplements and More Warned for Manufacturing Violations, Drug Claims
On December 17, 2014, the FDA issued a warning letter to Dandy Day Corporation, following a facility inspection which found the company's products, including Aloe Pearl, Alfalo, Aloespring, Crave Away, Original Supreme Energy, Ener "G," Can G, Flora G, Flora G Plus, and Garolic, to be adulterated ...
Recalls & Warnings
December 12, 2014
Seller of Omega-3 "Syrup" Warned for Manufacturing Violations and Drug Claims
On October 16, 2014, the FDA issued a warning letter to Mr.
Recalls & Warnings
December 12, 2014
Seller of B-12, Zinc, Echinacea and More Warned for Manufacturing Violations and Drug Claims
On November 14, 2014, the FDA issued a warning letter to Scientific Botanicals Company, Inc.
Recalls & Warnings
August 26, 2014
Maker of Herbal Supplements Warned for Manufacturing Violations and Drug Claims
On August 8, 2014, the FDA issued a warning letter to EnerHealth Botanicals, LLC following a facility inspection which found the company's products, including Parasite Purge Herbal Remedy, Black Walnut Extract, Bladder Cleanse Herbal Extract, Lung Renewal Herbal Remedy, EchinOsha and Daily Immune ...
Recalls & Warnings
October 21, 2014
Seller of Weight Loss Supplements Warned for Manufacturing Violations and Drug Claims
On October 7, 2014, the FDA issued a warning letter to YoungYou International, Inc.
Recalls & Warnings
October 16, 2014
Seller of Black Cohosh, Ginkgo and More Warned For Manufacturing Violations and Drug Claims
On April 2, 2014, the FDA issued a warning letter to Pure Herbs, Ltd.
Recalls & Warnings
March 28, 2011
Dangerously High Levels of Vitamins A and D in Product Prompt FDA Warning
On March 28, the U.S. FDA warned consumers to stop using Soladek, a vitamin-solution product marketed by Indo Pharma, S.A., of the Dominican Republic, because the product may contain dangerously high levels of vitamins A and D.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
January 03, 2004
FDA to Prohibit Sales of Dietary Supplements Containing Ephedra
On December 30, 2003, the U.S. Food and Drug Administration (FDA) issued a consumer alert of its forthcoming determination that dietary supplements containing ephedra present an unreasonable risk of illness or injury, and should not be consumed.
Recalls & Warnings
October 24, 2012
FDA Seizes Many Supplements from New York Company Due To Drug Claims
On October 23, 2012, U.S. Marshalls, acting on behalf of the FDA, seized dietary supplements and unapproved drugs from Confidence, Inc., a supplement manufacturer in Port Washington, N.Y. The products included dietary supplements Dr.
Recalls & Warnings
February 24, 2015
Seller of Antioxidant Water, Energy Drops Warned for Manufacturing Violations and Drug Claims
On February 18, 2015, the FDA issued a warning letter to Better Health Lab, Inc.
Recalls & Warnings
October 03, 2014
Seller of Green Tea, Prostate, Pain Supplements and More Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to AMS Health Sciences, LLC following a facility inspection which found the company's products, including saba ACE, UROPOWER, UROSure, Digest-Eze, Shark Cartilage, Colloidal Silver, and Mobilite to be adulterated because they were prepared, packed, ...
Recalls & Warnings
December 16, 2015
Maker of Red Yeast Rice, St. John's Wort, Valerian and More Warned for Manufacturing Violations, Drug Claims
On December 2, 2015, the FDA issued a warning letter to Nature's Health, LLC, following a facility inspection which found the company's product, including Ginkgo & Rhodiola, Blood Sugar Balance IV, Cinnamon Extract, Choles-Balance Red Yeast Extract, Lecithin, Milk Thistle Seed Extract, ...
Recalls & Warnings
September 11, 2015
Thirteen Manufacturers Ordered to Stop Selling Devil's Claw Supplements
On September 9, 2015, the New York State Attorney General ordered 13 manufacturers, including Thorne Research Inc., NBTY, Inc. (Puritan's Pride) Inc., and Now Foods, to stop selling devil's claw supplements which were found to contain the incorrect species of the plant.
Recalls & Warnings
June 27, 2017
Seller of Alpha Lipoic Acid, Cinnamon Supplements and More Warned For Manufacturing Violations
On May 1, 2017 the FDA issued a warning letter to Nature's Vision, Inc.
Recalls & Warnings
June 06, 2017
Seller of Herbal Supplements Warned for Manufacturing Violations, Drug Claims
On May 25, 2017 the FDA issued a warning letter to Life Rising Corporation, following a facility inspection which found the company's products, including Stomach Regulator, Fang Feng Formula, Skin Regulator, Regulate Liver, Circulation Regulator, Pancreas Support, Lung Regulator, Pure Tea, ...
Recalls & Warnings
May 30, 2017
Seller of Vitamin C, Calcium, Mushroom Supplements and More Warned for Manufacturing Violations
On May 12, 2017 the FDA issued a warning letter to VitaPurity Corporatio.
Recalls & Warnings
June 10, 2017
Seller of Prostate, Immune Supplements & More Warned for Manufacturing Violations
On May 25, 2017 the FDA issued a warning letter to The Herbalist, Inc.
Recalls & Warnings
May 30, 2014
Seller of Brain, Diabetes, Echinacea Supplements and More Warned for Drug Claims
On April 22, 2014, the FDA issued a warning letter to Mundo Natural Inc., following a review of the company's website which found statements made about supplements Neuro AD, Stop Diabetes, Stop High Blood Pressure, Morninga 7 and Purpurea Echinacea to be drug claims.
Recalls & Warnings
October 09, 2013
FDA Warns Consumers Not to Use OxyElite Pro As It Investigates Link With Hepatitis
On October 8, 2013, the FDA warned consumers not to buy or use USPLabs’ weight loss supplement OxyElite Pro because it has been linked with 24 cases of acute non-viral hepatitis in Hawaii.
Recalls & Warnings
September 07, 2002
FDA Sends Warning Letters to Seven Online Supplement Sellers For Therapeutic Claims
On August 27, 2002, the FDA posted seven "Cyber" letters recently issued from its Center for Food Safety and Applied Nutrition (CFSAN) to internet website operators promoting dietary supplement products that claim to diagnose, mitigate, treat, cure, or prevent a specific disease or class of ...
Recalls & Warnings
May 30, 2015
Seller of Omega-3, Probiotics & SuperFoods Warned for Manufacturing Violations, Drug Claims
On May 4, 2015, the FDA issued a warning letter to Dr. Dennis Black, LLC.
Recalls & Warnings
March 10, 2015
Maker of Magnesium, Calcium, Zinc, B Vitamins and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Complete H2O Minerals, following a facility inspection which found the company's products, including Sulfur Concentrate, Copper, Extra Strength Copper, Extra Strength Magnesium, Extra Strength Zinc, Platinum, Gold, Indium, Molybdenum, ...
Recalls & Warnings
October 30, 2014
Seller of Vitamin C, Iron and Detox Supplements Warned for Violations, Drug Claims
On October 16, 2014, the FDA issued a warning letter to Vitalab Co., Inc. (which manufactures and labels supplements for three distributors: V.E. Irons, Inc., Springreen Products, Inc., and Sonne's Organic Foods, Inc.
Recalls & Warnings
June 23, 2017
FDA Warns Seller of Menopause, Sexual Enhancement, Prostate Supplements and More For Manufacturing Violations
On May 26, 2017 the FDA issued a warning letter to Star Health & Beauty LLC, following a facility inspection which found the company's products, including Nu Essentials Royal Jelly Capsules, NuMan Male Enhancement Capsules, Star's Male Potency Tonic, NuGen HP, She Max HP, and V Max ...
Recalls & Warnings
November 07, 2015
Seller of "NaturalDoctor" Vitamin C, Echinacea and More Warned for Manufacturing Violations
On October 16, 2015, the FDA issued a warning letter Sound Healing Arts, PC, dba Grounds for Tea, LLC, following a facility inspection which found the company's products, including NaturalDoctor Vitamins C & K3, NaturalDoctor Goldenseal & Echinacea Plus, NaturalDoctor Centella ...
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
June 26, 2015
Seller of CoQ10, Anxiety Supplements and More Warned for Manufacturing Violations, Drug Claims
On June 6, 2015, the FDA issued a warning letter to Country Doctor Herbals, following a facility inspection which found the company's products, including Herbal Flu Be-Gone, Herbal Multi-Bac, Herbal Nervine, Herbal Pain-A-Way, Herbal Pancreas, Herbal Parasite Cleanse, Herbal Perfect ...
Recalls & Warnings
October 05, 2011
FDA Warns Nature's Rite of Promoting 8 Supplements as Drugs
The FDA published a Warning Letter dated September 19, 2011 to Nature's Rite, LLC stating that the firm’s online promotion of its various supplements for the treatment or prevention of various diseases cause these products to be considered unapproved drugs.
Recalls & Warnings
August 29, 2012
Herbal Supplement Company Warned For Medical Claims
On August 2, 2012, the FDA issued a warning to HSAC Enterprises, Inc. dba Kare-N-Herbs subsequent to a facility inspection and website review in May 2012 which found statements made about Kold Kare, Energy Kare and Tranquility Kare dietary supplements to constitute drug claims.
Recalls & Warnings
May 07, 2012
FDA Warns Doctor Promoting Own Supplements Online as Treatments
On April 18, 2012 the U.S. Food and Drug Administration (FDA) sent a Warning Letter to Jacob Teitelbaum, M.D. of Fatigued to Fantastic, LLC regarding the promotion that company's products on its website www.endfatigue.com.
Recalls & Warnings
January 04, 2012
Seller of "HCG Drops" for Weight Loss Warned by FDA and FTC of Violations
On December 21, 2011, the U.S. FDA and FTC sent a joint Warning Letter to hCG Drops LLC regarding its marketing of Slim Diet Drops. According to the letter, the product is considered an unapproved drug because it is promoted for drug-like uses on the company's website, including the following:
Recalls & Warnings
June 21, 2010
FDA Warns Parents to Be Careful Giving Vitamin D Drops to Infants
On June 15, 2010, the U.S. FDA (Food and Drug Administration) alerted parents and caregivers that some liquid Vitamin D supplement products are sold with droppers that could allow excessive dosing of Vitamin D to infants.
Recalls & Warnings
April 11, 2008
Update: Hazardous Levels of Selenium Confirmed by FDA in Two Supplements
On April 11, 2008, the FDA notified healthcare professionals and patients that it has found hazardous levels of selenium in samples of certain flavors of the dietary supplement products "Total Body Formula" and "Total Body Mega Formula.
Recalls & Warnings
July 01, 2003
Direct Marketers of Weight Loss, Impotence, and Arthritis Supplements Charged with Deceptive Claims
On July 1, 2003, the Federal Trade Commission (FTC) announced three enforcement actions against direct marketers of weight-loss products containing ephedra. The two settlements and one complaint, filed in U.S.





