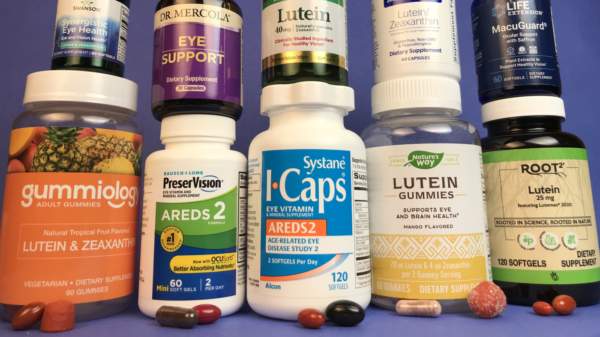
Reviews and Information for Vision Supplements
Vision Supplements with Lutein, Zeaxanthin & AREDS2 Formulas
Find the best vision supplements containing lutein and zeaxanthin. See tests of popular brands like PreserVision, ICaps, MacuGuard, MacuGuard, and more. Find out which supplements most closely resemble AREDS and AREDS2 formulas and see how products compare. Review the evidence for slowing the progression of age-related macular degeneration.
Vision Supplements with Lutein, Zeaxanthin & AREDS2 Formulas ReviewSearch term may appear only in full report available to members. Join now for full access.
Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

CL Answer
Which vitamins and minerals should someone over 70 take?
Find out which vitamin and mineral supplements men and women age 70 and older should take, recommended intakes for vitamin D, B6, B12, iron, plus multis, vision supplements, protein and more.

News Release
April 24, 2025
ConsumerLab Tests Identify Best Glycine Supplements
White Plains, NY, April 24, 2025 — Glycine supplements are promoted to improve sleep and mood, reduce symptoms of overactive bladder and chronic prostatitis, and for other benefits, but do they really work, and if so, which glycine products on the market are best?
CL Answer
I've been using a lutein supplement with 40 mg of lutein and am concerned that this dose may be too high. Are there known harmful side-effects?
Learn more about lutein side effects, proper dosage and safety in vision supplements.

News Release
December 28, 2023
10 Most Popular ConsumerLab Product Reviews of 2023
White Plains, New York, December 28, 2023 — In 2023, ConsumerLab tested more than two hundred products, adding to its extensive Product Reviews covering 150 categories of supplements, foods, and other health products. Among these, the following 10 were the most popular in 2023:
Clinical Update
6/07/2024
Supplement for Dry Eye?
A new "dry eye supplement" decreased symptoms of dry eye in a recent study - but was it enough to stop using lubricating drops? Find out more in the What It Does section of our Vision Supplements Review, which includes our Top Picks among vision supplements.
Clinical Update
5/12/2013
Best Supplements for Vision (Macular Degeneration)
A major new study revealed what combination of supplements is best for slowing progression of macular degeneration -- and which don't seem to help. Get the details, plus quality ratings on over 20 vision supplements in the updated Vision Supplements Review >>
Clinical Update
7/15/2017
Lutein May Reduce Eye Strain and Headaches
Taking a combination of lutein and other carotenoids may help reduce eye strain, fatigue, headaches and sleep problems associated with extended time spent in front of computer screens. Get the details in the "What It Does" section of our Vision Supplements Review. The Review includes our tests and quality comparisons of popular vision supplements.
Clinical Update
7/13/2018
Fruit for the Eyes
Can consuming certain foods or beverages reduce the risk of developing age-related macular degeneration (AMD) — a common eye disease? Yes, according to a recent study. Get the details in the food section of the Vision Supplements Review. (Also see our Top Rated vision supplements.)
Clinical Update
4/30/2019
Saffron for Vision
Saffron, which contains carotenoids, may improve visual acuity in people with age-related macular degeneration (AMD) according to a recent study. For details, see the ConsumerTips section of the Vision Supplements Review. Also see which vision supplements most closely resemble the ideal formula to slow the progression of AMD.
Clinical Update
10/31/2020
Fish Oil & Vitamin D for Macular Degeneration?
Can taking fish oil and vitamin D reduce the risk of age-related macular degeneration (AMD)? See what a large study found in the Eye Disease section of the Fish Oil Supplements Review. Also see the Vision Supplements Review for our tests of vision supplements, including those that most closely resemble the AREDS2 formula and our Top Picks among products.
Clinical Update
11/30/2021
Caution With Vitamin Supplements
A woman who, for years, had taken a popular vision supplement developed anemia and low white blood cell and platelet counts. What was the connection? Find out in the Concerns and Cautions section of our Vision Supplements Review. Also see our Top Picks among vision supplements.
Clinical Update
8/29/2023
Counterfeit Vision Supplement
Counterfeit versions of a major brand of eye vitamin have been sold on Amazon. For details, see the Update in our Vision Supplements Review, which includes our Top Picks among vision supplements.
Also see: How to Avoid Buying Counterfeit Vitamins and Supplements
Clinical Update
1/25/2022
Goji Berries for the Eyes?
Goji berries are a good source of zeaxanthin, an anti-oxidant compound that, like lutein, may help protect the retina of eye. But does eating goji berries improve a measure of macular health (macular optical density) or improve vision? See what recent research showed in the Getting Lutein and Zeaxanthin from Food section of our Vision Supplements Review. Also see our Top Picks among vision supplements.
CL Answer
Which supplements are helpful for age-related macular degeneration (AMD)?
Find out which vitamins & supplements are helpful for macular degeneration (AMD), such as lutein, zeaxanthin, and others used in the AREDS studies.

CL Answer
Does taking vitamin C (1,000 mg) deplete copper in the body?
Find out if taking vitamin C supplements can deplete copper levels in the body and cause copper deficiency. ConsumerLab.com's answer explains.

News Release
July 13, 2023
Best Vision Supplements According to ConsumerLab Tests
White Plains, New York, July 13, 2023 — Studies show that supplements containing lutein, zeaxanthin, zinc, and other ingredients can help slow the progression of age-related macular degeneration, which is the leading cause of blindness in adults over age 60.
News Release
February 13, 2023
Saffron Supplements Vary Widely in Key Compounds, According to ConsumerLab Tests
White Plains, New York, February 13, 2023 — Saffron extracts are promoted to treat mood, improve cognition and vision, and even reduce appetite.
Recalls & Warnings
September 12, 2024
Root Bioscience Warned for CBD Claims
On August 30, 2024, the FDA issued a warning letter to Root Bioscience Brands, LLC dba Naternal following a review of the company's websites, which found statements made about the company's products, including CBD Oils Move CBD+CBG Oil, Rest CBD+CBN Oil, Nurture Broad Spectrum ...
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Clinical Update
12/23/2024
Turmeric & Vision
A long-term study found that people using turmeric (curcumin) supplements were far less likely to develop age-related macular degeneration than non-users. For details, see our Turmeric Supplements Review, which includes our Top Picks among turmeric and curcumin supplements.
Also see our Vision Supplements Review, which includes our Top Pick among AREDS2 vision formula supplements.
Clinical Update
Clinical Update
7/22/2024
Ocuvite for Macular Degeneration?
Do Ocuvite supplements provide the same ingredients shown to slow the progression of macular degeneration? Find out in our Vision Supplements Review.
Clinical Update
2/23/2024
Vision Supplement Discontinued
Our original Top Pick among supplements to slow age-related macular degeneration has been discontinued. We discuss other options in the Top Picks section of our Vision Supplements Review.
Clinical Update
3/01/2024
Leaky Pills
A problem with a popular vision supplement was reported by a CL member and there have been similar reports from other customers. For details, see the Vision Supplements Review.
Clinical Update
5/14/2024
Best Form of Lutein?
Is one form of lutein more effective in raising blood levels of lutein than another? See what a recent study found in the ConsumerTips section our Vision Supplements Review, which also explains the best way to take lutein to improve absorption. Also see our new Top Pick vision supplement for slowing age-related macular degeneration.
Clinical Update
3/20/2025
Light Therapy for Macular Degeneration?
Use of a multiwavelength light device may slow progression and improve vision among people with age-related macular degeneration (AMD), according to a recent study, but not all light devices show a benefit. Get the details in our article about the health benefits and safety of red and near infrared light.
Also see our Vision Supplements Review, which includes our Top Pick for slowing macular degeneration.
Clinical Update
3/31/2025
Cocoa for Aging Eyes?
Did taking cocoa extract slow macular degeneration (AMD) in older people? Find out what a recent study showed in the Vision section of our Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review, which includes our Top Picks among supplements, cocoas and mixes, dark chocolates and chips, and cacao nibs.
Also see our Top Picks AREDS formulas to slow the progression of AMD in our Vision Supplements Review.
Clinical Update
9/19/2024
Propolis for Vision?
Propolis is found in some eye formulas, but is there good evidence that is helps with conditions such as glaucoma, cataracts, or retinal damage? Find out in our Vision Supplements Review, which includes our Top Picks among lutein and AREDS formula supplements.
Also find out if propolis has shown benefit for fatty liver disease or arthritis.
Clinical Update
10/21/2024
Pistachio Eye Benefits?
Did eating pistachios, a natural source of lutein, improve macular pigment density in the retinas of people with low density levels (a risk factor for age-related macular degeneration)? Find out what a recent study showed in the Macular Degeneration section of our Vision Supplements Review, which includes our Top Pick lutein and AREDS2 supplements
Also see: Do pistachios provide melatonin?
Clinical Update
8/12/2015
Lutein, Zeaxanthin & Meso-Zeaxanthin for Macular Degeneration
A recent study comparing three different lutein and zeaxanthin formulas found each significantly increased macular pigmentation in men and women with early age-related macular degeneration. Get the details, including dosage, and our tests of popular lutein and zeaxanthin products, in the Vision Supplements Review »
Clinical Update
10/05/2019
Better Memory With Lutein?
Lutein is often taken as a supplement to slow the progression of age-related macular degeneration. However, a study in college students suggests that supplementation with lutein may also improve aspects of memory and cognitive functioning. For details, see the What It Does section of our Vision Supplements Review.
Clinical Update
10/01/2017
Does Lutein Help?
Lutein is an antioxidant that helps protect the retina of the eye and can slow the progression of macular degeneration. But does taking lutein help if you already have normal macular density? Two studies have "looked" at this question (including one published this week) and, you can "see" the answer in the "What It Does" section of Vision Supplements (Lutein & Zeaxanthin) Review >>
Clinical Update
1/16/2018
Lutein and Zeaxanthin Improve Memory
Lutein and zeaxanthin may help to slow the progression of age-related macular degeneration in people with low blood levels of lutein. A new study suggests these compounds may have another benefit -- improving memory in some people. Get the details in the "What It Does" section of the Vision Supplements Review >> (Also see our top choices among products.)
Clinical Update
6/21/2015
Lutein and Visual Processing
Lutein and zeaxanthin are known to help certain people who suffer from macular degeneration, an eye disease. A new study suggests that they may also improve visual processing, perhaps through effects outside the eye. For more details of the study, including dosage, as well as our tests of supplements, see the Review of Vision Supplements with Lutein and Zeaxanthin Review >>
Clinical Update
2/25/2017
Lutein for Stress?
Supplementing with lutein can help some people protect against macular degeneration of the eye. A new study suggests it may also reduce stress. For details, see the "What It Does" section of the Review of Vision Supplements with Lutein and Zeaxanthin (which includes our tests of products) >>
Clinical Update
6/03/2022
Is Lutein Safe?
Was adding lutein and zeaxanthin to a popular supplement for age-related macular degeneration safe and effective? Find out what a major study found in the What It Does section of our Vision Supplements Review.
Clinical Update
6/10/2022
Supplements for Farsightedness?
Can a supplement improve vision in older people who develop trouble seeing up-close (i.e., presbyopia or age-related farsightedness)? See the study results for a supplement that included lutein, astaxanthin, bilberry and DHA in our Vision Supplements Review.
Clinical Update
12/02/2022
Vision Supplement Concern
We were asked to evaluate EyePromise AREDS 2 Plus Zinc-Free by several CL members. We have not tested this product, but have concerns about its formula and dosing, as explained in our Vision Supplements Review.
Clinical Update
3/17/2023
Lycopene and Lutein Absorption
Carotenoids, such as lycopene and lutein, are best absorbed when taken with a meal with oils -- but the type of oil matters, according to a recent study that compared the effects of olive oil and coconut oil. See the details in the What to Consider When Using section of our Lycopene Supplements Review, which includes our Top Pick among lycopene supplements.
Also see our Top Picks among lutein and vision supplements and our answer to the question: Which supplements should be taken with food?”
CL Answer
Are antioxidant supplements good or bad for you?
Learn more about whether antioxidants like astaxanthin, beta-carotene, coenzyme Q10, curcumin, resveratrol, selenium, vitamin A, vitamin C and vitamin E are safe and if they help prevent chronic diseases.

Recalls & Warnings
February 17, 2022
Seller of L-Theanine, Lutein, Noopept and More Warned for Drug Claims
On February 4, 2022, the FDA issued a warning letter to Crystal Clear Supplements following a review of the company’s website and social media which found statements about Tianeptine Sodium Powder, Tianeptine Sulfate Powder, PEA Capsules, Phenibut Capsules, Synephrine Powder, Noopept ...
Recalls & Warnings
June 30, 2022
FDA Warns Seller of Vision and Allergy Supplements
On May 26, 2022, the FDA issued a warning letter to Golden Lab LLC following an inspection of the website, which found statements about the company’s DoctoRx’s Optimal Formula Ocular Pressure & Optic Nerve Support Formula Ocular Health Capsule, DoctoRx’s Optimal Formula ...
CL Answer
How much calcium do you need each day, what forms are best, and how much is too much?
Learn about the recommended daily intake of calcium from food & supplements and why taking too much can be harmful. Also, learn about the forms of calcium that are available and which are best.

Recalls & Warnings
November 09, 2023
Zazzee Naturals Warned for MSM Eye Drop Claims
On October 30, 2023, the FDA issued a Warning Letter to Dexterity Health, LLC DBA Zazzee Naturals following review of the company’s Amazon storefront, which found statements about the company’s Liquid MSM Drops to be drug claims.
Product Review
Saffron Supplements Review
See How Saffron Supplements Compare and What They Do

Product Review
Vitamin A Supplements Review, Including Beta-Carotene and Cod Liver Oil
Find the Best Vitamin A Supplement. Best Quality Vitamin A Supplements Identified, Including Cod Liver Oil.

Recalls & Warnings
December 01, 2021
FDA Warns Seller of Saffron Supplements
On November 18th, 2021, the FDA issued a warning letter to Saffron Health Sciences following a review of the company's website, social media, and Amazon product listings, which found statements made about the company's products Crocin Rich, Crocin Rich II, and Crocin Rich ...
CL Answer
Can castor oil eye drops improve or dissolve cataracts? Is castor oil or tea tree oil helpful for other eye conditions such as blepharitis or dry eye?
Can castor oil eye drops help cataracts or for other conditions such as blepharitis? Find out if castor oil eye drops are safe to use, and if they help for other conditions. ConsumerLab.com's answer explains.

Recalls & Warnings
September 14, 2023
FDA Warns CVS, Walgreens, Similasan & Others for Eye Drop Violations
On September 11, 2023, the FDA issued Warning Letters to the following eight sellers of homeopathic and other types of eye drops regarding the products noted in italics due to a variety of violations of FDA regulations, most notably that they were marked with claims suggesting that they could cure, ...
CL Answer
Do any supplements help prevent or improve cataracts?
Find out which supplements may help with cataracts, including selenium and vitamins A, B1, B3, C, and E.

CL Answer
Do any supplements help prevent or reduce eye floaters?
Find out eye floaters (vitreous floaters) be treated with supplements like vitamin C, lutein, ginkgo, milk thistle, collagen or hyaluronic acid, or with products such as VitreousHealth.
CL Answer
Does TA-65 Astragalus Extract Increase Telomere Length and Longevity?
TA-65 Astragalus Extract is promoted to increase telomere length. Find out if there is evidence for this, if this increases lifespan or longevity, plus safety, side effects and cost.

CL Answer
Can supplements, natural eye drops, or lifestyle modifications improve symptoms of dry eye?
Can supplements such as fish oil, flaxseed oil or curcumin (from turmeric) help treat dry eyes? Find out, including formulas and doses that have shown benefit. Also, learn if drinking more water or using eye drops containing castor oil, N-acetylcarnosine or aloe can help with dry eye.

Clinical Update
Clinical Update
4/29/2018
Dark vs. Milk Chocolate for Vision?
A recent study compared the effects on visual acuity of eating a bar of dark chocolate versus milk chocolate. Find out which was better in the Vision section of the Dark Chocolates, Cocoa Powders, and Cacao Nibs Review. (Also see our product tests, comparisons, and Top Picks.)
CL Answer
How to Reduce Lightheadedness and Dizziness When Standing Up (Orthostatic Hypotension)
Learn about movements that can reduce lightheadedness and dizziness that can occur from a drop in blood pressure upon standing (initial orthostatic hypotension). Plus find out if supplements such as vitamin D and CoQ10 may help.

CL Answer
Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
Find out which supplements help improve memory, brain function and cognition, including fish oil, some B vitamins, cocoa, and curcumin. ConsumerLab's answer explains the evidence for supplements promoted to help with brain function and cognition.
Recalls & Warnings
February 06, 2023
Recalled Eye Drops Linked to Vision Loss and One Death
On February 2, 2023, Global Pharma Healthcare issued a nationwide recall of its Artificial Tears Lubricant Eye Drops due to potential contamination with bacteria that can cause serious infection, blindness, and death.
CL Answer
Do brain games and apps really work and improve memory?
Find out if brain training games & apps, like Lumosity & BrainHQ, really work to train the mind and improve memory. ConsumerLab.com's answer explains.

CL Answer
Are There Supplements That Help Treat POTS (Postural Orthostatic Tachycardia Syndrome)?
Find out which supplements and lifestyle changes may be helpful for people with Postural Orthostatic Tachycardia Syndrome (POTS).

Clinical Update
10/10/2023
PEA for Glaucoma?
Does PEA (palmitoylethanolamide) reduce intraocular pressure and slow loss of vision due to glaucoma? Find out in our article about PEA.
Clinical Update
12/02/2022
Astaxanthin for Better Vision?
Did astaxanthin improve the visual acuity of people who spend a lot of time in front of a computer screen? See the results of a recent study in the What It Does section of the Astaxanthin Supplements Review.
Clinical Update
3/11/2022
How Vitamin A Deficiency Can Affect the Eyes
Vitamin A deficiency was linked to eye floaters and loss of peripheral vision in a recently reported case. Get the details, and learn about other symptoms of vitamin A deficiency, in the What It Does section of our Vitamin A Supplements Review. Also see our Top Picks among vitamin A supplements.
See our answer to the question: Do any supplements help prevent or reduce eye floaters?
Clinical Update
6/28/2022
COVID-19 Tests for Sight Impaired
People with blindness or low vision can now order more accessible COVID-19 tests from the government. Get the details.
Clinical Update
12/15/2018
Pycnogenol for Prostate?
Pycnogenol, a branded form of pine bark extract, is promoted for a variety of uses, including improvement of circulation, cognition, joint pain and vision. It was recently given to men with benign prostatic hyperplasia (BPH) to see if it would improve urinary symptoms. Find out if it worked, and learn if it helps with other conditions, in our updated answer to the question: What is Pycnogenol and does it work? >>
Clinical Update
4/10/2025
TA-65 for Vision or Heart Health?
Does TA-65 (astragalus root extract) help people with macular degeneration or reduce blood pressure, cholesterol, or inflammation? Find out in our updated article about TA-65.
Clinical Update
5/10/2024
New Top Pick for Vision
We have tested and chosen a new Top Pick among supplements to slow the progression of age-related macular degeneration. Get the details. Also, be aware that some formulas contain too much zinc, which has been reported to cause anemia.
Clinical Update
10/21/2024
Melatonin for Vision?
Does taking melatonin slow the progression of age-related macular degeneration? Find out what studies show in the What It Does section of our Melatonin Supplements Review, which includes our Top Picks for melatonin.
Clinical Update
11/18/2024
Urolithin A for Vision?
Can urolithin A supplementation help prevent age-related macular degeneration, a common cause of blindness? Find out what research suggests in our article about urolithin A.
Clinical Update
6/07/2024
Melatonin & Vision
A strong link between melatonin use and lower risk of age-related macular degeneration was shown in a large U.S. study. For details, see the What It Does section of our Melatonin Supplements Review, which includes our Top Picks for melatonin.
Recalls & Warnings
November 29, 2023
Dr. Berne’s Whole Health Products Warned for Contaminated Eye Drop Products, Drug Claims
On November 22, 2023, the FDA issued a Warning Letter to Dr. Berne’s Whole Health Products following review of the company’s website, which found statements about the company’s Dr. Berne’s MSM Eye Drops 5%, Dr. Berne’s MSM Eye Drops 15%, and Dr.
Recalls & Warnings
December 07, 2023
Belmont Eyecare Warned for Colloidal Silver, MSM, and Castor Oil Eye Drop Claims
On December 1, 2023, the FDA issued a Warning Letter to Belmont Eyecare LLC, following a review of the company’s website, which found statements about the company’s Colloidal Silver Eye Drops, MSM Eye Drops, Organic Daytime Oil Eye Drops, Organic Daytime Oil Eye Drops Small, Organic ...
Recalls & Warnings
February 28, 2024
Microbial Contamination Found in LightEyez MSM Eye Drops
On February 15, 2024, the FDA issued a Warning Letter to LightEyez Limited after testing by the agency found the company’s LightEyez.com MSM Eye Drops V2.0 — Eye Repair (MSM Eye Repair Drops) to have microbial contamination in samples of the drops.
News Release
October 22, 2019
Best Vision Supplements Identified by ConsumerLab
White Plains, New York, October 22, 2019 — Supplements containing lutein and other ingredients can slow the progression of age-related macular degeneration, the leading cause of vision loss and blindness in adults over 60 years of age.
Recalls & Warnings
August 25, 2022
Oregon’s Wild Harvest Warned by FDA for Manufacturing Violations
On July 8, 2022, the FDA issued a warning letter to Oregon’s Wild Harvest, Inc.
CL Answer
What are the benefits of grape seed extract?
Learn about the health benefits of grape seed extract, and find out it improves venous insufficiency, leg swelling, swelling after surgery, or can help to lower blood pressure.

Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
February 14, 2017
Life Extension Warned for Drug Claims
On February 1, 2017, the FDA issued a warning letter to Life Extension Foundation Buyers Club, Inc.
Recalls & Warnings
August 13, 2016
Seller of Omega-3, Ginkgo, Vision Supplements & More Warned for Drug Claims
On July 22, 2016, the FDA issued a warning letter to Viva Nutraceuticals (J & E International Corporation) following a review of the company's website and promotional literature, which found statement made about its products, Eyestonia, Ginkgo Biloba 60mg, Golden Omega-3 Max, ...
Recalls & Warnings
April 25, 2013
Vision Supplement Company Warned For Drug Claims
On March 15, 2013, the FDA issued a warning letter to Synticare Corp. because statements made on the company's website about certain supplements, including Maximum Lutein, AREDS 1, AREDS 1 Smokers, AREDS 2, Combo AREDS 1 and 2, and Combo Smokers AREDS 1 and 2, were found to be drug claims.
Recalls & Warnings
May 15, 2016
Seller of Vision Supplements Warned for Drug Claims
On April 28, 2016, the FDA issued a warning letter to Macular Health, LLC.
Recalls & Warnings
March 10, 2015
Seller of Vision Supplements Warned for Manufacturing Violations, Drug Claims
On February 26, 2015, the FDA issued a warning letter to Biosyntrx, following a facility inspection which found the company's products, including BioTears Oral Gel Caps, ZoOmega-3 Concentrated Pharmaceutical-Grade Fish Oil, EpiCor A Nutrient-dense High Metabolite Immunogen, Sight C+ Mineral ...
Recalls & Warnings
January 16, 2015
CVS Sued for Eye Health Supplement Claims
A class-action lawsuit has been filed against CVS for promoting the company's Advanced Eye Health supplement as comparable to the formula used in the large National Institutes of Health (NIH) eye disease study known as AREDS2, despite the fact that it does not contain the same ingredients.
Recalls & Warnings
July 10, 2021
FDA Warns Seller of Omega XL
On June 31, 2021 the FDA issued a warning letter to Great Healthworks, Inc. following a review of the company's website, which found statements made about Omega XL and ProbioticXL supplements to be drug claims.
Recalls & Warnings
May 08, 2021
FDA Warns Seller of Vitamins B12, C, D, and K
On April 7, 2021, the FDA issued a warning letter to Unived Inc following a review of the company's websites, which found statements made about the company's products Unived brand Colox, CalDveg, B12+D3, and D3+K2-7 to be drug claims.
Recalls & Warnings
May 08, 2021
Seller of Immune Bio Green Cell Warned by FDA
On March 30, 2021, the FDA issued a warning letter to Immune & Genetics Protocols, LLC following a review of the company's websites, which found statements made about the company's product Immune Bio Green Cell to be drug claims.
Recalls & Warnings
July 22, 2022
FDA Warns Seller of Vision, Calcium & Magnesium Supplements and More
On June 14, 2022, the FDA issued a warning letter to New Sun Inc. following inspection of the company’s website which found statements about the company’s products to be drug claims.
News Release
October 26, 2018
Best Vitamin A Supplements, Including Cod Liver Oils, Identified by ConsumerLab
White Plains, New York, October 26, 2018 — Vitamin A is necessary to maintain good vision, skin, and immune functioning, and supplementation can be beneficial for certain conditions — although most people get enough from food and getting too much vitamin A can be dangerous.
News Release
February 24, 2017
Vitamin D Supplements Maintain Top Spot in Popularity, as Probiotics Surpass Multivitamins to #4 Behind Fish Oil and CoQ10 in Latest ConsumerLab.com Survey
White Plains, New York, February 24, 2017 — A recent survey of over 9,505 people who use dietary supplements shows the most popular dietary supplement to be vitamin D, followed by fish oil, CoQ10, probiotics, and multivitamins.
Recalls & Warnings
August 05, 2016
Seller of Fertilix Warned By FDA
On July 22, 2016, the FDA issued a warning letter to CellOxess LLC, following a review of the company's websites, social media pages and marketing literature which found statements made about Fertilix Preconceptual, Fertilix Low Dose and Fertilix Max to be drug claims.
Recalls & Warnings
July 23, 2016
Seller of GlucoCor Warned for Drug Claims
On June 30, 2016, the FDA issued a warning letter to MC-COR, LLC, following a review of the company's website and Facebook page which found statements made about its GlucoCor capsules to be drug claims.
News Release
December 17, 2015
ConsumerLab.com Focuses on Vision Supplements, Spotting the Best and the Worst
White Plains, New York, December 17, 2015 — Supplements containing lutein and other ingredients have been shown to slow deterioration of the retina in people with age-related macular degeneration.
Recalls & Warnings
August 28, 2023
Dr. Berne’s MSM and Castor Oil Eye Drops Recalled Due to Bacterial and Fungal Contamination
On August 26, 2023, Dr. Berne’s Whole Health Products issued a recall of all lots of its MSM DROPS 5%, 15% Solution, Dr. Berne’s Organic Castor Oil Eye Drops and Dr. Berne’s MSM MIST 15% Solution due to the risk of bacterial and fungal contamination.
Recalls & Warnings
December 19, 2024
Jarritos Coconut Water Recalled
On November 4, 2024, Tipp Distributors, Inc. issued a recall all lot codes of Jarritos Coconut Water (17.5 fl oz cans) because the airtight seal on the lid of the cans may be compromised.
Recalls & Warnings
July 23, 2025
FDA Warns Seller of Allegro Eye Drops, Tea Tree Oil Eyelid Wipes, and More
On July 9, 2025, the FDA issued a Warning Letter to Scope Health Inc.
Recalls & Warnings
September 08, 2025
FDA Warns Two Sellers of Homeopathic Eye Drops
On August 25, 2025, the FDA issued Warning Letters to Supply Center USA and Homeopathic Educational Services (dba Homeopathic Family Medicine) following reviews of the companies’ websites, which found statements about certain "homeopathic" eye drop products to be drug claims.
Recalls & Warnings
November 09, 2023
InnoMark Warned for Manufacturing & Misbranding Violations
On September 1, 2023, the FDA issued a Warning Letter to InnoMark, Inc.
Recalls & Warnings
August 12, 2024
FDA Warns "Apricot Power" Apricot Seeds Contain Toxic Compound
On May 24, 2024, the FDA warned consumers not to consume three Apricot Power bitter apricot seed products because they were found to contain the toxic compound amygdalin.
Recalls & Warnings
June 24, 2024
Canned Coffee Recalled Due to Botulism Risk
On June 17, 2024, Snapchill LLC recalled its canned coffee products because they have the potential to grow the toxic bacterium Clostridium botulinum.
Recalls & Warnings
September 26, 2012
Maker of Eye Health Supplements Warned for Drug Claims
On September 18, 2012, the FDA issued a warning letter to EyeScience Labs, L.L.C. because statements made about the company's Macular Health Formula, Dry Eye Formula and Diabetic Vision Formula supplements were found to constitute drug claims.
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
October 18, 2017
Total Body Nutrition Warned for Banned Stimulant in Supplements
On September 28, 2017, the FDA issued a warning letter to Total Body Nutrition following a facility inspection which found some of the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
June 07, 2017
FDA Warns Seller of Probiotic and Omega-3 Supplements for Manufacturing Violations
On May 22, 2017, the FDA issued a warning letter to BioTE Medical, LLC following a facility inspection which found its products, including BioTE DIM, BiotTE Probiotic, BioTE Iodine Plus, and BioTE Omega 3 to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
October 25, 2016
FDA Warns Maker of Sublingual Melatonin and Progesterone Cream
On October 12, 2016, the FDA issued a warning letter to Threshold Enterprises, Ltd.
Recalls & Warnings
October 18, 2016
Seller of Sublingual Vitamins Warned for Drug Claims
On August 31, 2016, the FDA issued a warning letter to Bio-Stasis International, Inc., following a review of the company's website which found statements made about its sublingual tablets, ViraPress, Vitamin D-3 and Vitamin B-12. to be drug claims.
Recalls & Warnings
September 07, 2016
Seller of Herbal Formulas for Cholesterol, Prostate & More Warned For Drug Claims
On July 29, 2016, the FDA issued a warning letter to Healing-Scents following a review of the company's website, which found statements made about its products, Heart Herbs, Cholesterol Regulation Herbs, Diabetes Regulation Herbs, Prostate Healer Herbs, High Blood Pressure Herbs, ...
Recalls & Warnings
January 24, 2013
Excessive Lead and Mercury Found In Chinese Herbal Supplements
On January 23, 2013, Health Canada warned consumers that certain batches of the Chinese dietary supplements [W.S.
Recalls & Warnings
January 24, 2013
Food for Health Warned for Manufacturing Violations and Drug Claims on Supplements
On October 5, 2012, the FDA issued a warning letter to Food for Health International, LLC following a facility inspection which found the company's products, including Activz brand Vitamin D, Potassium Iodine, Organic Vitamin C, Whole 9 (a fruit and vegetable meal replacement shake) Control and VMA ...
Recalls & Warnings
January 21, 2017
Seller of "Cancer Kits," Alpha Lipoic Acid Supplements and More Warned for Drug Claims
On December 22, 2016, the FDA issued a warning letter to Northern Health Products, Inc. following a review of the company's websites, www.northernhealthproducts.com and www.petdca.
Recalls & Warnings
January 17, 2017
Seller of Heart, Senior and Teen Supplements Warned For Drug Claims
On December 7, 2016, the FDA issued a warning letter to Esteem Products Ltd following a review of the company's website, which found statements made about its products, Cardio Life, Total Man, Total Woman, Senior Total Man, Senior Total Woman , and Total Teen to be drug ...
News Release
July 31, 2013
Watch Out for Fake Bilberry Supplements, Cautions ConsumerLab.com
WHITE PLAINS, NEW YORK July 31, 2013 — Extracts of bilberry, a cousin of blueberry, may help improve vision and have other health benefits, but some bilberry supplements may not contain real bilberry extract -- the type used in most clinical studies. ConsumerLab.
Recalls & Warnings
November 10, 2022
Adam’s Polishes Hand Sanitizer Recalled Due to Toxic Methanol
On November 5, 2022, Adam’s Polishes, LLC issued a nationwide recall of 20 lots of Adam’s Polishes Hand Sanitizer following FDA testing, which found the presence of methanol in one lot.
Recalls & Warnings
November 21, 2022
6 Supplement Companies Warned by FDA for Making Cholesterol Claims
On May 4, 2022, the FDA issued warning letters to six supplement companies following review that found statements made on company websites and Walmart purchase pages suggesting the products could lower cholesterol to be drug claims, which are not permitted for dietary supplements.
News Release
October 17, 2012
ConsumerLab.com Tests and Compares Vision Supplements - Guides Consumers Through a Variety of Ingredients and Formulas
White Plains, New York — October 17, 2012 — Can a supplement improve your vision or prevent it from deteriorating? "The evidence shows that some supplements may help improve vision in people with macular degeneration and others can slow its progression," says Tod Cooperman, M.D.
News Release
December 20, 2011
Choose zinc supplements carefully -- ConsumerLab.com finds only some have dosage proven to shorten colds, reduce eye disease
White Plains, New York — December 20, 2011 — Can zinc supplements shorten colds and reduce the progression of advanced macular degeneration? "Yes, but not all supplements provide a dosage that has been proven effective." says Tod Cooperman, M.D., President of ConsumerLab.com.
News Release
December 16, 2009
"Eye health" supplements vary widely in formula but product quality is high according to ConsumerLab.com
"Eye Health" Supplements Vary Widely in Formula But Product Quality is High According to ConsumerLab.com
News Release
December 09, 2008
Tests of zinc supplements by ConsumerLab.com show only some provide dosage proven to shorten colds, reduce eye disease. Lead contamination found in one product.
WHITE PLAINS, NEW YORK — DECEMBER 9, 2008 — Zinc supplements can shorten colds and reduce the progression of advanced macular degeneration, among other uses. But a new report by ConsumerLab.
News Release
July 12, 2007
ConsumerLab.com finds lutein & zeaxanthin supplements vary widely in strength but most meet ingredient claims — Right amount of antioxidant may help prevent macular degeneration and improve vision
WHITE PLAINS, NEW YORK — July 12, 2007 — ConsumerLab.com announced today that all but one of the thirteen lutein and zeaxanthin supplements it recently chose to test met quality standards.
Recalls & Warnings
February 12, 2025
Trader Joe’s, Van Camp’s, H-E-B, and Genova Canned Tuna Recalled
On February 7, 2025, Tri-Union Seafoods recalled select canned tunas sold by Trader Joe’s, Van Camp’s, H-E-B, and Genova, due to a manufacturing defect that compromises the integrity of the “easy open” pull tab on the can.
Recalls & Warnings
December 21, 2016
Seller of 5-HTP, Chromium, Curcumin and More Warned for Drug Claims
On December 1, 2016, the FDA issued a warning letter to Aurora Health and Nutrition following a review of the company's website, which found statements made about its products, 5-HTP, Alpha Lipoic Acid, the Argentyn 23 product line, Artecin 90 Vegetarian Capsules, ...
Recalls & Warnings
February 13, 2013
Manufacturer of Heart, Multivitamin, Cranberry, and Longevity Supplements Warned For Adulteration and Drug Claims
On January 29, 2013, the FDA issued a warning letter to M.D.R. Fitness Corp. following a facility inspection which found violations of good manufacturing practices which cause the company's products to be adulterated.
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
July 28, 2022
FDA Warns Three Manufacturers of Sanitizer for Manufacturing and Misbranding Violations
Between July 8 and July 21, the FDA issued warning letters to three companies selling hand sanitizer products that violate Current Good Manufacturing Practices (cGMP), are adulterated and misbranded, and for refusal to allow the agency to access and copy company records.
Recalls & Warnings
September 21, 2023
Moor Herbs, Inc Warned for Manufacturing Violations, Drug Claims
On August 1, 2023, the FDA issued a Warning Letter to Moor Herbs, Inc.
Recalls & Warnings
August 16, 2023
FDA Warns Sun Ten Laboratories for Manufacturing Violations, Drug Claims
On April 7, 2023, the FDA issued a warning letter to STPCA Inc. dba Sun Ten Laboratories because products were found to be adulterated because they were prepared, packed, or held under conditions that do not meet Current Good Manufacturing Practices (CGMP).
Recalls & Warnings
April 27, 2023
Seller of Magtein, Veggie Caps, & More Warned for Drug Claims, Manufacturing Violations
On March 8, 2023, the FDA issued a warning letter to Spartan Enterprises Inc., dba Watershed Wellness Center, after a review of the company’s website found statements about its Dr. Bob’s Naturals Spirulina, Dr. Bob’s Naturals Magtein, Dr.
Recalls & Warnings
May 23, 2013
Seller of Sexual Enhancement, Cholesterol, Resveratrol Supplements and More Warned For Drug Claims
On May 2, 2013, the FDA issued a warning letter to Alternative Health Supplements, following a review of the company's website, which found statements made about several dietary supplements, including Regenerect, Alligin, Astaxanthin Advantage, HDL Cholesterol Management, Resveratrol, Coral Calcium ...
Recalls & Warnings
January 25, 2021
Clover Leaf Sardines Recalled Due to Risk of Botulism
On January 22, 2021, Clover Leaf Seafoods Corp.
Recalls & Warnings
November 10, 2020
FDA Warns Immusist for Drug Claims
On October 16, 2020, the FDA issued a warning letter to Immusist, LLC following a review of the company's website, which found statements made about the company's products IMMUSIST Original and IMMUSIST Natural to be drug claims.
Recalls & Warnings
November 10, 2020
Seller of Cholesterol-Lowering Supplements Warned for Drug Claims
On October 29, 2020, the FDA issued a warning letter to Natural Sprout Co.
Recalls & Warnings
April 06, 2022
Mandalorian and Mickey Mouse Hand Sanitizers Recalled Due to Benzene, Methanol
On April 1, 2022, Best Brands Consumer Products, Inc.
Recalls & Warnings
August 08, 2022
Recall of Oral Magnesium Laxatives Expanded
On August 3, 2022, Plastikon Healthcare, LLC issued a recall of multiple oral suspension products due to microbial contamination.
Recalls & Warnings
September 28, 2022
FDA Warns Muscle Sports for Joint Health, Immune & Workout Supplement, Vitamin C, Elderberry, and Other Claims
On September 23, 2022, the FDA issued a warning letter to Muscle Sports Products, LLC following inspection of the company’s websites which found statements about company products, including Join Revolution Capsules, IMMUNITY + Powder, Rhino Rampage PUMPED Capsules, Attack Pre-Workout ...
Recalls & Warnings
September 11, 2020
M Hand Sanitizer Recalled
On September 9, 2020, Medek, LLC recalled M Hand Sanitizer Alcohol Antiseptic 80% 128 oz/3,785 mL because it may contain methanol, which is toxic when absorbed through the skin or ingested. The product may also have a sub-potent ethanol content, which leads to a lack of efficacy.
Recalls & Warnings
September 05, 2020
Bio aaa Advance Hand Sanitizer Recalled
On September 3, 2020, AJR Trading LLC recalled one lot of bio aaa Advance Hand Sanitizer because different lots of the product may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
September 01, 2020
Mislabeled Open Book Extracts Hand Sanitizers Recalled
On August 29, 2020, Open Book Extracts recalled Always Be Clean Hand Sanitizer and Just Hand Sanitizer because, according to the product labels, they contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
August 26, 2020
Asiaticon Hand Sanitizers Recalled
On August 25, 2020, Asiaticon SA de CV recalled V-Klean Hand Sanitizer Gel, Medically Minded Hand Sanitizer Gel, and Protz Real Protection Antibacterial Hand Sanitizer because they may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
August 25, 2020
Florence Morris Antiseptic Hand Sanitizer Recalled
On August 17, 2020, Grupo Asimex De Mexico Sa De CV recalled Florence Morris Antiseptic Hand Sanitizer because it may contain methanol, which is toxic when absorbed through the skin or ingested. The products also may have a sub-potent ethanol content, which leads to a lack of efficacy.
Recalls & Warnings
August 20, 2020
Yacana Hand Sanitizer Recalled
On August 18, 2020, Grupo Yacana México S.A.S De C.V. recalled Yacana Hand Sanitizer because it may contain methanol, which is toxic when absorbed through the skin or ingested. The products also may have a sub-potent ethanol content, which leads to a lack of efficacy.
Recalls & Warnings
August 13, 2020
FDA Warns Soluciones Cosmeticas for Hand Sanitizers That May Contain Toxic Ingredient
On August 4, 2020, the FDA issued a warning letter to Soluciones Cosmeticas, SA de CV which after FDA testing found samples of the company's BERSIH Antiseptic Alcohol 70% Topical Solution Hand Sanitizer and BERSIH HAND SANITIZER GEL Fragrance Free to contain methanol, a toxic ...
Recalls & Warnings
August 10, 2020
Gelbac T Antibacterial Handgel Recalled
On August 6, 2020, Incredible Products Sa De Cv recalled Gelbac T Antibacterial Handgel because it may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
July 29, 2020
Herbacil Antiseptic Hand Sanitizer Recalled
On July 27, 2020, Broncolin S.A. de C.V. recalled Herbacil Antiseptic Hand Sanitizer 70% Alcohol because it may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
July 28, 2020
FDA Warns Eskbiochem for Hand Sanitizers That May Contain Toxic Ingredient
On July 23, 2020, the FDA issued a warning letter to Eskbiochem SA de CV which found the company's products, including ClearCare Nogerm ADVANCED HAND SANITIZER and LAVAR GEL HAND SANITIZER, to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
July 17, 2020
Two More Hand Sanitizers That May Contain Toxic Ingredient Recalled
Update: (8/10/20) Soluciones Cosméticas has recalled additional products that may contain methanol, as listed in red below.
Recalls & Warnings
July 07, 2020
All Clean Hand Sanitizer Recalled
On July 6, 2020, ITECH 361 recalled All Clean Hand Sanitizer, Moisturizer and Disinfectant because it may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
June 27, 2020
Hand Sanitizer That May Contain Toxic Ingredient is Recalled
Update: (6/29/20) Saniderm has extended the recall of hand sanitzer nationwide.
Recalls & Warnings
December 24, 2020
Wash-Free Hand Sanitizer With Potentially Toxic Ingredient Recalled
On December 23, 2020, Shane Erickson, Inc.
Recalls & Warnings
November 27, 2020
FDA Warns Seller of Potentially Toxic Hand Sanitizer
On November 19, 2020, the FDA issued a warning letter to Liq-E S.A. De C.V. because laboratory tests showed that their product Optimus Lubricants Instant Hand Sanitizer contains methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
December 31, 2020
Herbacil Hand Sanitizer Contains Dangerous Ingredient
On December 21, 2020, the FDA issued a warning letter to Broncolin, S.A. de C.V. because laboratory tests showed that their product HERBACIL Antiseptic Hand Sanitizer contains methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
February 09, 2021
Three More Hand Sanitizers Contain Toxic Ingredient
Between February 3 and 5, 2021, the FDA issued warning letters to three sellers of hand sanitizers because laboratory tests showed that their products contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
January 28, 2021
FDA Issues Import Alert on Potentially-Dangerous Hand Sanitizers from Mexico
On January 26, 2021, the FDA announced that all alcohol-based hand sanitizers from Mexico will be placed on a countrywide import alert to limit the flow of potentially dangerous products into the US.
Recalls & Warnings
March 26, 2021
Nine Banned Stimulants Found in Workout, Weight Loss Supplements
Recent analysis of 17 brands of sports/energy and weight loss supplements sold in the U.S. found nine prohibited stimulants formulated into eight different combinations, and none of these combinations have been studied in people.
Recalls & Warnings
March 16, 2021
Albek de Mexico Hand Sanitizer Contains Dangerous Ingredient
On March 11, 2021, the FDA issued a warning letter to Albek de Mexico S.A. de C.V. because laboratory tests showed that their product NEXT ADVANCED ANTIBACTERIAL HAND SANITIZER contains methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
March 09, 2021
FDA Warns Sellers of Three More Hand Sanitizers, One with Toxic Ingredient
On March 1, 2021, the FDA issued warning letters to three sellers of hand sanitizers because laboratory tests showed that their products do not contain the amount of ethanol as stated on their labels. The products are MEDICALLY MINDED Hand Sanitizer Gel from Plasticos Las Palmas, S.A. de C.
Recalls & Warnings
February 19, 2005
Seller of Eye Supplement Purporting to Restore Vision and Eliminate "Floaters" Settles FTC Charges
On February 15, 2005, the Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, have settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular ...
Recalls & Warnings
May 20, 2005
Seller of "Ocular Nutrition" Supplement Settles FTC Charges
On March 16, 2005, The Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular Nutrition) – ...
Recalls & Warnings
June 23, 2020
Nine Hand Sanitizers May Contain Toxic Ingredient That Can Cause Serious Illness or Death
Update: (7/14/20) The FDA has warned consumers not to use forty-six more hand sanitizers that may contain methanol.
Recalls & Warnings
July 06, 2020
FDA Warns Five More Hand Sanitizers May Contain Toxic Ingredient
Update: (7/14/20) The FDA has warned consumers not to use forty-six more hand sanitizers that may contain methanol.
Recalls & Warnings
August 04, 2020
Two More Companies Recall Potentially Toxic Hand Sanitizers
Between July 30 and 31, 2020, two more companies recalled hand sanitizers because they may contain methanol, which is toxic when absorbed through the skin or ingested:
Recalls & Warnings
August 17, 2020
SkinGuard24 Hand Sanitizer Recalled
On August 14, 2020, SG24 LLC recalled SkinGuard24 — All Day Hand Sanitizer because it is labeled as containing methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
October 01, 2020
Cleaner Hand Sanitizer Recalled
On September 8, 2020, DMM VISSION, S.A. DE C.V. recalled five lots of Cleaner Hand Sanitizer because they may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
March 02, 2021
FDA Warns Makers of Four Hand Sanitizers, Two With Toxic Ingredient
Between February 18 and 25, 2021, the FDA issued warning letters to four sellers of hand sanitizers because laboratory tests showed that their products do not contain the amount of ethanol as stated on their labels.
Recalls & Warnings
May 15, 2021
Toxic Hand Sanitizer Recalled
On May 11, 2021 Dibar Nutricional S. de R.L. de C.V. issued a recall of various lots of DIBAR Labs Hand Sanitizer and ProtectoRx Hand Sanitizer because they were confirmed to contain methanol.
Recalls & Warnings
February 19, 2019
Alaskan Smoked Silver Salmon in Jars and Cans Recalled Due to Risk of Botulism
On February 15, 2019, Smoked Alaska Seafoods, Inc. recalled all jars and cans of Smoked Silver Salmon because it has the potential to be contaminated with Clostridium botulinum, a bacterium which can cause life-threatening illness or death.
Recalls & Warnings
February 17, 2010
FTC Warns of Deceptive Claims with Children's Omega-3 Supplements
On February 17, 2010, the Federal Trade Commission (FTC) announced that it had sent letters to 11 companies that promote various omega-3 fatty acid supplements, telling them they should review their product packaging and labeling to make sure they do not violate federal law by making baseless ...
Recalls & Warnings
June 27, 2017
Seller of Alpha Lipoic Acid, Cinnamon Supplements and More Warned For Manufacturing Violations
On May 1, 2017 the FDA issued a warning letter to Nature's Vision, Inc.
Recalls & Warnings
October 27, 2004
Some Supplements Can Damage Eyes At High Doses and with Topical Use
As reported by Reuters Health on October 21, 2004, an article in the current issue of the American Journal of Ophthalmology suggests that some herbal remedies and nutritional supplements can damage the eyes.
Recalls & Warnings
July 21, 2020
Optimus Instant Hand Sanitizer Recalled
On July 20, 2020, LIQ-E S.A. de C.V. recalled Optimus Instant Hand Sanitizer because it may contain methanol, which is toxic when absorbed through the skin or ingested.
Recalls & Warnings
August 24, 2012
Protein Shots, Drinks and Gelatin Recalled For Potential Botulism Contamination
On August 17, 2012, Protica Inc.
Recalls & Warnings
December 08, 2009
Warning on Acai Berry Supplements Spiked with Drug
On December 8, 2009, Health Canada (the Canadian health agency) advised consumers not to use certain Acai Berry products after a large number of shipments of adulterated products were stopped at the border.
Recalls & Warnings
July 01, 2013
Teas Recalled Due To Potential Botulism Contamination
One June 28, 2013, Harmony Chai issued a voluntary recall of its Concentrated Black Spiced Chai and Decaffeinated Rooibos Chai bottled tea drinks, because they have the potential to be contaminated with Clostridium botulinum.
Recalls & Warnings
May 06, 2017
Herbal Teas Recalled Due To Botulism Risk
On May 1, 2017, U.S. Deer Antler Ex. & Imp. of Los Angeles, California, issued a recall of various herbal teas because they have the potential to be contaminated with Clostridium botulinum. This bacterium can cause botulism, a potentially fatal form of food poisoning.
Recalls & Warnings
July 18, 2017
Seller of "Super Strength Prostate Formula," Acai Berry Supplements & More Warned for Drug Claims
On June 30, 2017, the FDA issued a warning letter to Nature's Health Company, LLC, following a facility inspection and review of the company's website, www.natureshealthcompany.
Recalls & Warnings
July 11, 2017
FDA Warns Dr. Carolyn Dean of Drug Claims Made for Magnesium, Calcium & Other Mineral Supplements
On June 27, 2017, the FDA issued a Warning Letter to Dr. Carolyn Dean of New Capstone, Inc.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
October 18, 2005
FTC Stops False Claims about HGH Oral Spray
On October 18, 2005, the Federal Trade Commission (FTC) announced that, at its request, a federal court issued a temporary restraining order against marketers of oral sprays that supposedly contain human growth hormone (HGH) to stop them from making alleged false and deceptive claims and from ...
Recalls & Warnings
August 31, 2007
FTC Stops Spammers Selling Bogus Hoodia and Human Growth Hormone
On August 23, 2007, the Federal Trade Commission (FTC), announced that spammers must stop sending unwanted and illegal e-mail messages about hoodia weight-loss products and human growth hormone anti-aging products the FTC alleges don't work.