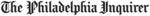Product Reviews and Information for Eye Health
Search term may appear only in full report available to members. Join now for full access.
Product Review
Vision Supplements Review (with Lutein, Zeaxanthin & AREDS2 Formulas)
Find the Best Vision Supplement Based Our Tests
News Release
January 23, 2025
ConsumerLab Tests Identify Best Black Currant, Borage, Evening Primrose Flax and Hemp Oils
White Plains, NY, January 23, 2025 — Black currant, borage, evening primrose, flax, and hemp oils contain omega-3, -6, and/or -9 fatty acids that, when replacing saturated fats in the diet, may help reduce cardiovascular risk.
Product Review
Vitamin E Supplements Review
Find the Best Vitamin E Supplement. Tests and Reviews of Popular Vitamin E Supplements & CL's Top Picks.
Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

CL Answer
Which supplements are helpful for age-related macular degeneration (AMD)?
Find out which vitamins & supplements are helpful for macular degeneration (AMD), such as lutein, zeaxanthin, and others used in the AREDS studies.

CL Answer
Does red and near infrared light therapy reduce pain, improve skin or cognition, or have other benefits? Is it safe?
Red light and near infrared light therapy devices are promoted for numerous conditions, including acne, fibromyalgia, pain, cognitive function and other uses. Find out if these devices work and if they are safe.

News Release
July 13, 2023
Best Vision Supplements According to ConsumerLab Tests
White Plains, New York, July 13, 2023 — Studies show that supplements containing lutein, zeaxanthin, zinc, and other ingredients can help slow the progression of age-related macular degeneration, which is the leading cause of blindness in adults over age 60.
Product Review
Vitamin A Supplements Review, Including Beta-Carotene and Cod Liver Oil
Find the Best Vitamin A Supplement. Best Quality Vitamin A Supplements Identified, Including Cod Liver Oil.

Product Review
Digestive Enzyme Supplements Review
Big Differences in Strength Found Among Digestive Enzyme Supplements. CL Tests Reveal Which Are Best.

News Release
November 27, 2017
Flaxseed and Other Popular Omega 3 & 6 Oil Supplements Tested, But Not All Brands Pass -- Tests of Black Currant Oil, Borage Oil, Evening Primrose Oil, Flaxseed Oil, and Hemp Oil Supplements
White Plains, New York, November 27, 2017 — Flaxseed oil and other popular seed oil are popular supplements that provide omega-3 and omega-6 fatty acids and may help with inflammatory conditions. But which seed oil supplements are the best? To find out, ConsumerLab.
CL Answer
Do any supplements help prevent or improve cataracts?
Find out which supplements may help with cataracts, including selenium and vitamins A, B1, B3, C, and E.

CL Answer
Can supplements, natural eye drops, or lifestyle modifications improve symptoms of dry eye?
Can supplements such as fish oil, flaxseed oil or curcumin (from turmeric) help treat dry eyes? Find out, including formulas and doses that have shown benefit. Also, learn if drinking more water or using eye drops containing castor oil, N-acetylcarnosine or aloe can help with dry eye.

CL Answer
What is lactoferrin and will it really strengthen my immune system?
Info about lactoferrin supplements, clinical evidence for strengthening the immune system, preventing or treating colds, gut health and treating H.
CL Answer
Can castor oil eye drops improve or dissolve cataracts? Is castor oil or tea tree oil helpful for other eye conditions such as blepharitis or dry eye?
Can castor oil eye drops help cataracts or for other conditions such as blepharitis? Find out if castor oil eye drops are safe to use, and if they help for other conditions. ConsumerLab.com's answer explains.

Product Review
Lavender and Tea Tree Essential Oils Review
Oils Tested for Authenticity and Purity

CL Answer
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
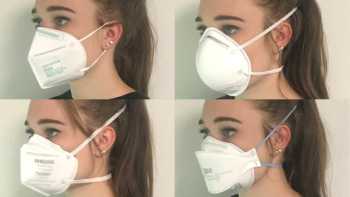
CL Answer
What are the side effects of glucosamine and chondroitin?
Learn the side effects of glucosamine and chondroitin supplements for joint health and joint pain, including stomach upset, headache, and many others. ConsumerLab.com's answer explains.

Product Review
Manuka Honey Review
Find the Best Manuka Honey Based on Quality, Taste, and Value. See our Top Picks.

CL Answer
Does glucosamine raise "bad" LDL cholesterol?
Learn more about the link between glucosamine and cholesterol, including evidence from clinical studies.

CL Answer
Do any supplements help prevent or reduce eye floaters?
Find out eye floaters (vitreous floaters) be treated with supplements like vitamin C, lutein, ginkgo, milk thistle, collagen or hyaluronic acid, or with products such as VitreousHealth.
Recalls & Warnings
January 16, 2015
CVS Sued for Eye Health Supplement Claims
A class-action lawsuit has been filed against CVS for promoting the company's Advanced Eye Health supplement as comparable to the formula used in the large National Institutes of Health (NIH) eye disease study known as AREDS2, despite the fact that it does not contain the same ingredients.
Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

Product Review
Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, Boswellia, Collagen and Turmeric)
18% of Joint Health Supplements Failed to Pass Our Tests. See Which Passed or Failed, and Our Top Picks.

CL Answer
Are light boxes effective for preventing and treating seasonal affective disorder (SAD), also known as "winter depression," or circadian rhythm disorders such as jet lag? Which light boxes are best?
Learn about the benefits and safety of light boxes (including who is most likely to benefit), how to use these devices, which commercially available products appear to meet the technical requirements, and who should not use light boxes.

Clinical Update
7/22/2025
Lactoferrin for Dry Eye?
Lactoferrin is a natural component of tears, but can taking lactoferrin as a supplement relieve symptoms of dry eye? Find out in our article about the health benefits of lactoferrin. Also learn about other supplements, eye drops, and lifestyle modifications for dry eye.
Clinical Update
6/02/2013
Glucosamine May Increase Eye Pressure
New research suggests that people with glaucoma or intraocular hypertension could have worsening eye pressure if they take a glucosamine supplement. Get the details (along with our tests of glucosamine and other joint health supplements) in the updated Glucosamine/Joint Health Supplements Review >>
CL Answer
L-Tyrosine: Health Effects and Safety
Tyrosine has been evaluated for conditions including physical and mental stress, phenylketonuria (PKU), depression, and attention deficit-hyperactivity disorder (ADHD). Find out if it works and if it's safe.

Clinical Update
1/25/2022
Goji Berries for the Eyes?
Goji berries are a good source of zeaxanthin, an anti-oxidant compound that, like lutein, may help protect the retina of eye. But does eating goji berries improve a measure of macular health (macular optical density) or improve vision? See what recent research showed in the Getting Lutein and Zeaxanthin from Food section of our Vision Supplements Review. Also see our Top Picks among vision supplements.
Clinical Update
5/28/2019
Glucosamine & Eye Pressure
Glucosamine may increase eye pressure in people with glaucoma or intraocular hypertension. But does it have this effect in people who don't have these conditions? See what a new study found in the Concerns and Cautions section of the Joint Health Supplements Review. Also see our Top Picks for glucosamine and chondroitin.
Clinical Update
6/19/2025
Red Light Devices & Eye Protection
Find out when eye protection is recommended with red light therapy in the Concerns and cautions section of our article about red light therapy. Also learn about possible health benefits of red light therapy.
CL Answer
Are L-carnosine supplements helpful and safe?
Learn about the potential health benefits of L-carnosine, as well as possible safety concerns, drug interactions and cost.

CL Answer
What is hesperidin, what is it used for and is it safe?
Learn about hesperidin, including whether its beneficial for heart health, problems with circulation, stroke, cognitive function, depression, cancer and other conditions, and find out if it is safe.

CL Answer
Do any supplements reduce wrinkles, increase skin elasticity, or tighten the skin?
Learn about collagen, biotin, CoQ10, cocoa, soy isoflavones, phytoceramides, and other supplements for reducing wrinkles and improving aging skin.

CL Answer
What are the health benefits of chlorophyll and chlorophyllin, and are they safe?
Chlorophyll supplements are promoted for reducing body odor, preventing cancer, removing toxins from the body, improving digestive conditions and other uses, but do they work and are they safe? Find out and learn if such supplements actually contain chlorophyll.

CL Answer
What is EpiCor? Does it really "boost" the immune system and prevent colds?
EpiCor supplement info, including evidence from clinical studies on immune function and cold/flu prevention, safety, and price.

News Release
September 13, 2016
Best Fish Oil? ConsumerLab.com Tests 44 Popular Omega-3 Supplements and Identifies Its Top Picks
White Plains, New York, September 13, 2016 — Fish oil and omega-3 fatty acid supplements containing EPA and DHA are among the most popular supplements, and clinical studies suggest a wide range of potential benefits, from improving mental health to treating inflammatory disease.
Clinical Update
8/23/2022
Can-C Eye Drops for Dry Eye?
A product called Can-C Eye Drops has been promoted for improving dry eye as well as reducing the risk of cataracts. Does the evidence support this? Find in our updated CL Answer about supplements for dry eye.
Clinical Update
6/24/2025
Dry Eye: Do Warm Compresses Help?
Are warm compresses or microwaveable eye masks useful for relieving dry eye? Find out in our article about dry eye, which includes information about supplements, natural eye drops, and lifestyle modifications that may help.
Clinical Update
7/18/2025
Drops for Dry Eye
We’ve added information about common OTC eye drops to our article about supplements and eye drops for dry eye, including which ingredients are best for different types of dry eye and the pros and cons of preservatives.
CL Answer
Is it safe to consume fish oil as a long-term food supplement?
Learn more about fish oil supplement safety including long-term use, side effects, and more.

Clinical Update
7/28/2018
Dry Eye, Fish Oil and Contact Lenses
Taking fish oil can help people with dry eye and it was recently studied for dry eye associated with wearing contact lenses. Find out what the recent study found and learn more about fish oil for dry eye in the What It Does section of the Fish Oil & Omega-3 Supplements Review. (Also see our Top Picks among fish oil supplements.)
Clinical Update
8/19/2022
Supplements for Dry Eye
We added information about the following topics to our article about supplements and lifestyle modification for dry eye:
- Does coffee or caffeine supplements improve dry eye?
- Does krill or algal oil have similar effects as fish oil for dry eye disease?
Clinical Update
8/08/2023
Castor Oil Eye Drops
Are castor oil eye drops helpful for dry eye, blepharitis, or cataracts, and are they safe? Find out in our article about castor oil eye drops.
Clinical Update
7/08/2025
Is Castor Oil Safe for Eyes?
Some eye drops contain castor oil, but is it safe to apply regular castor oil to the eyes? Find out in our article about using castor oil for cataracts, dry eye, and blepharitis.
Clinical Update
11/28/2023
Enzymes for Eye Floaters
Does supplementing with digestive enzymes reduce eye floaters and improve sight? See what a recent study showed in the Eye Floaters section of our Digestive Enzymes Supplements Review.
Also see: Do any supplements help prevent or reduce eye floaters?
Clinical Update
5/21/2024
MSM Drops for Dry Eye?
Should you consider eye drops containing MSM for dry eye? Find out in our article about "natural" treatments and supplements for dry eye.
CL Answer
I was told I have a MTHFR gene mutation. Do I need to take methylated forms of B vitamins, such as methylfolate and methylcobalamin?
MTHFR gene mutation can impact B vitamin bioavailability. Learn more about this and which deficiencies are more likely to occur.

Clinical Update
6/24/2015
Fish Oil for Dry Eye
A large study of computer-using young men and women experiencing dry eye found that fish oil significantly improved symptoms. Benefits have also been reported in postmenopausal women with dry eye. Details, including dosage, are found in the "What It Does" section of the Fish Oil Supplements Review >>
Clinical Update
9/21/2021
Enzyme Supplements for Eye Floaters?
Can enzyme supplements (bromelain, papain and ficin) reduce eye floaters? Find out what a recent study suggests in the What They Do section of our Digestive Enzyme Supplements Review.
Also see our answer to the question: Do any supplements help prevent or reduce eye floaters?
Clinical Update
3/11/2022
How Vitamin A Deficiency Can Affect the Eyes
Vitamin A deficiency was linked to eye floaters and loss of peripheral vision in a recently reported case. Get the details, and learn about other symptoms of vitamin A deficiency, in the What It Does section of our Vitamin A Supplements Review. Also see our Top Picks among vitamin A supplements.
See our answer to the question: Do any supplements help prevent or reduce eye floaters?
Clinical Update
8/16/2022
Water for Dry Eye?
Does drinking extra water help with dry eye? Find out in our updated article about dry eye -- which includes information about supplements and lifestyle habits than can help.
Clinical Update
6/14/2022
Fish Oil For Dry Eye?
Can taking fish oil reduce dry eye in older men and women? Find out what a recent study showed in the What It Does section of our Fish Oil Supplements Review.
Also see our CL Answer about Supplements for Dry Eye.
Clinical Update
9/07/2022
Supplement for Eye Floaters?
Did a supplement containing vitamin C, zinc, and other antioxidant ingredients help reduce eye floaters in a recent study? Find out in our article: Do any supplements help prevent or reduce eye floaters?
Clinical Update
9/16/2022
Gluten & Dry Eye?
A CL member commented that their chronic dry eye went away after eliminating gluten from their diet. Is there clinical evidence that this can help? Find out in our article about supplements and lifestyle modifications for dry eye.
Clinical Update
11/22/2022
Maqui Berry for Dry Eye?
Can supplementing with maqui berry extract (in products from LifeExtension, NOW, Swanson, and other brands) improve symptoms of dry eye? Find out what research suggests in our updated CL Answer about supplements for dry eye.
Clinical Update
11/14/2023
Manuka Honey for Dry Eye?
Is manuka honey helpful for dry eyes when applied in an eye drop? Find out what research has shown in our article about manuka honey.
Also, find out if mixing manuka honey into hot beverages deactivates its key compounds.
Clinical Update
6/07/2024
Supplement for Dry Eye?
A new "dry eye supplement" decreased symptoms of dry eye in a recent study - but was it enough to stop using lubricating drops? Find out more in the What It Does section of our Vision Supplements Review, which includes our Top Picks among vision supplements.
Clinical Update
7/15/2025
Pineapple for Eye Floaters?
Does eating pineapple reduce eye floaters? Find out in the Eye Floaters section of our Digestive Enzyme Supplements Review.
Clinical Update
5/22/2025
Avoiding Eye Irritation from Sunscreens
Some sunscreens can cause eye irritation. Find out which types of sunscreen are least likely to cause eye irritation in our article about safe and effective sunscreens.
Clinical Update
6/19/2025
Dry Eye: Do Blue Light Glasses Help?
Does wearing blue light glasses improve symptoms of dry eye when using digital devices? Find out what a recent study showed in our article about supplements, drops, and lifestyle modifications for dry eye.
Clinical Update
5/17/2024
Can Omega-3 Help with Dry Eye?
Did taking an omega-3 fatty acid supplement for 3 months improve dry eye symptoms? Find out in the Dry Eye section of our Fish Oil/Omega-3 Supplements Review, which includes our Top Picks among omega-3 supplements.
CL Answer
Are antioxidant supplements good or bad for you?
Learn more about whether antioxidants like astaxanthin, beta-carotene, coenzyme Q10, curcumin, resveratrol, selenium, vitamin A, vitamin C and vitamin E are safe and if they help prevent chronic diseases.

Clinical Update
11/30/2021
Benefits of Flaxseed Oil
Find out if flaxseed oil helps with eye floaters in our updated answer to the question: Do any supplements help prevent of reduce eye floaters?
CL Answer
What is Pycnogenol, does it work, and is it the same as other pine bark extracts?
Learn more about Pycnogenol, including evidence from clinical studies on blood clots, osteoarthritis, cognition, vision, tinnitus, and the skin and safety.

CL Answer
Does PrimeBiome Improve Aging Skin?
Is there evidence that PrimeBiome or its ingredients improve the appearance of skin, and is it worth its price? Find out.

Clinical Update
12/14/2021
Fish Oil for Dry Eye?
Does supplementing with fish oil, alone or in combination with other oils, improve symptoms of dry eye? See the results of the latest study in the What It Does section of our Fish and Marine Oil Supplements Review. Also see our Top Picks among fish oil supplements.
Clinical Update
9/22/2020
Fish Oil and Eye Bleeding
Can high-dose fish oil increase the risk of bleeding in the eye? Get the details of a recently reported case in the Concerns and Cautions section of our Fish Oil Supplements review. Also see our Top Picks for fish oil.
Clinical Update
7/15/2017
Lutein May Reduce Eye Strain and Headaches
Taking a combination of lutein and other carotenoids may help reduce eye strain, fatigue, headaches and sleep problems associated with extended time spent in front of computer screens. Get the details in the "What It Does" section of our Vision Supplements Review. The Review includes our tests and quality comparisons of popular vision supplements.
Clinical Update
8/04/2013
Supplement Helps Dry Eye
A supplement containing omega-6 (from black currant seed oil) and omega-3 fatty acids (from fish oil) was recently shown to improve symptoms of dry eye. Get the details about this formula (plus our test results of other omega-3 and -6 fatty acids supplements) in the updated Black Currant, Borage, Evening Primrose, and Flaxseed Oils Review >>
Clinical Update
6/21/2015
Lutein and Visual Processing
Lutein and zeaxanthin are known to help certain people who suffer from macular degeneration, an eye disease. A new study suggests that they may also improve visual processing, perhaps through effects outside the eye. For more details of the study, including dosage, as well as our tests of supplements, see the Review of Vision Supplements with Lutein and Zeaxanthin Review >>
Clinical Update
4/14/2018
Fish Oil for Dry Eye?
A major study evaluated the effects of supplementing with the omega-3 fatty acids EPA and DHA in people with dry eye. Get the results and details in the What It Does section of the Fish Oil Supplements Review. (Also see our Top Picks among fish oil supplements).
Clinical Update
7/13/2018
Fruit for the Eyes
Can consuming certain foods or beverages reduce the risk of developing age-related macular degeneration (AMD) — a common eye disease? Yes, according to a recent study. Get the details in the food section of the Vision Supplements Review. (Also see our Top Rated vision supplements.)
Clinical Update
11/01/2019
Eye Risk With High-Dose Niacin
Very high doses of niacin are sometimes used to lower high cholesterol, but there are risks -- including damage to the eye, as shown in a recent case. Get the details in the Niacin section of the B Vitamin Supplements Review.
Clinical Update
5/05/2025
Collagen for Eye Wrinkles?
Did taking a popular type of collagen reduce eye wrinkles in a recent study? Find out in our Collagen Supplements Review, which includes our Top Pick collagen supplements for skin and for joints.
Clinical Update
6/30/2025
Eyedrop Lawsuit Settlement
Consumers who purchased certain homeopathic eyedrops can submit a claim as part of a class-action lawsuit settlement. Get the details in our article about supplements, natural eye drops, and lifestyle modifications for dry eye.
Clinical Update
8/30/2022
Tea Tree Oil Toxicity
Is tea tree oil safe to use around the eye? Be careful -- it can potentially cause eye injury.
There is also concern that tea tree oil can cause growth of breast tissue in young boys. Learn more about this in the Cautions and Concerns section of our Lavender and Tea Tree Essential Oils Review.
News Release
December 17, 2015
ConsumerLab.com Focuses on Vision Supplements, Spotting the Best and the Worst
White Plains, New York, December 17, 2015 — Supplements containing lutein and other ingredients have been shown to slow deterioration of the retina in people with age-related macular degeneration.
News Release
December 10, 2014
ConsumerLab.com Identifies Best Zinc Products for Colds, Eyes, and Zinc Deficiency -- Popular Zinc Supplements and Lozenges Tested and Compared
White Plains, New York, December 10, 2014 — "Zinc supplements have been shown to shorten colds and reduce the progression of advanced macular degeneration, but not all products provide the dosage proven effective, as well as pass criteria for supplement quality," says Tod Cooperman, M.D.
News Release
April 09, 2014
Best Fish Oil? ConsumerLab.com Testing Finds Many High Quality Supplements, But Pitfalls Exist
White Plains, New York, August 10, 2014 — Recent laboratory tests of fish oil, krill oil, algal oil, calamari oil, and green-lipped mussel oil supplements by ConsumerLab.com revealed quality problems with four out of 30 supplements selected for review.
News Release
December 16, 2013
What's Really in Turmeric Supplements and Spices? You May Not Want to Know -- 33% of Turmeric/Curcumin Supplements Fail ConsumerLab.com's Review; Filth Found in Spices
White Plains, New York, December 16, 2013 — Supplements containing the herb turmeric or its key constituent, curcumin, are popular treatments for inflammatory diseases. ConsumerLab.
News Release
October 17, 2012
ConsumerLab.com Tests and Compares Vision Supplements - Guides Consumers Through a Variety of Ingredients and Formulas
White Plains, New York — October 17, 2012 — Can a supplement improve your vision or prevent it from deteriorating? "The evidence shows that some supplements may help improve vision in people with macular degeneration and others can slow its progression," says Tod Cooperman, M.D.
News Release
August 22, 2012
Contamination and Other Problems Found in Fish Oil Supplements -- Large Review by ConsumerLab.com Reveals Excessive PCBs or Mislabeling in Over 30% of Omega-3 Supplements
August 22, 2012 — The quality of fish oil / omega-3 supplements varies across brands, according to recent tests by ConsumerLab.com, which independently reports on the quality of health products. Analyses of 35 products selected and purchased by ConsumerLab.
News Release
December 20, 2011
Choose zinc supplements carefully -- ConsumerLab.com finds only some have dosage proven to shorten colds, reduce eye disease
White Plains, New York — December 20, 2011 — Can zinc supplements shorten colds and reduce the progression of advanced macular degeneration? "Yes, but not all supplements provide a dosage that has been proven effective." says Tod Cooperman, M.D., President of ConsumerLab.com.
CL Answer
What is the best sunscreen based on safety and efficacy?
We’ve boiled down the latest research about mineral and chemical sunscreens, including product tests, to help you choose the best sunscreen (which will depend on whether you expect to be in or out of the water). We also list popular sunscreens that have, or have not, been found to contain cancer-causing compounds (benzene and benzophenone).

CL Answer
Is ingesting colloidal silver helpful for any condition and is it safe to use?
Find out if there is evidence showing colloidal silver can help to treat infections, boost the immune system, help with arthritis and more, plus find out if colloidal silver can cause blue skin or other side effects.

CL Answer
Does ergothioneine help prevent age-related problems such as impaired cognition, Alzheimer’s disease, Parkinson’s disease or cataract, and is it safe?
Learn about ergothioneine and its potential use for cognitive impairment, dementia, Alzheimer's disease, Parkinson's disease, cataract, or kidney disease, as well as its safety and cost of supplements containing this ingredient.

Clinical Update
7/18/2024
Red Light Devices – More Answers
We've added more information to our recent article about red near infrared light devices about based on questions received:
- Vitiligo: Does red light help with vitiligo?
- Eye conditions: Is red light effective and safe for treating eye conditions , such as macular degeneration?
- Joint Pain: Is Move+ Pro by Kineon cleared by the FDA for reducing joint pain?
- Cleared by FDA? What is the difference between a device that has been "cleared" by the FDA as opposed to "approved" by the FDA?
- Product status: What is the FDA status of Omnilux Contour Face Mask, TenDlite, PainAway, and FibroLux?
- Devices vs. Sunshine? Since sunshine includes the wavelengths from red and near infrared light devices, why not just get sun exposure?
Recalls & Warnings
July 23, 2025
FDA Warns Seller of Allegro Eye Drops, Tea Tree Oil Eyelid Wipes, and More
On July 9, 2025, the FDA issued a Warning Letter to Scope Health Inc.
CL Answer
What are the health benefits of coconut oil and medium chain triglycerides, such as those used in Bulletproof Coffee?
Learn the health benefits of coconut oil and medium chain triglycerides (MCTs), including evidence from Bulletproof Coffee, clinical studies on obesity, skin, and Alzheimer's disease.

News Release
September 21, 2011
Not all vitamin A supplements pass ConsumerLab.com tests -- New report reviews beta-carotene and cod liver oil supplements
White Plains, New York — September 21, 2011 — Recent tests of vitamin A supplements by ConsumerLab.com found one to contain only 54.3% of its listed amount of vitamin A. Another product mislabeled alpha carotene as vitamin A.
CL Answer
Is it better to take fish oil, flaxseed oil -- or both?
Fish oil and flaxseed oil -- how they are different, what they are used for, if and how they can help with arthritis, depression, lowering cholesterol & more.

Clinical Update
1/28/2022
Symptoms of Vitamin A Deficiency
Although not common, be aware of signs of vitamin A deficiency involving the eyes, skin, and nails, as exemplified by a recent case. Get the details (including medical conditions which can cause it) in the What It Does section of our Vitamin A Supplements Review. Also see our Top Picks among vitamin A supplements.
Clinical Update
10/31/2020
Fish Oil & Vitamin D for Macular Degeneration?
Can taking fish oil and vitamin D reduce the risk of age-related macular degeneration (AMD)? See what a large study found in the Eye Disease section of the Fish Oil Supplements Review. Also see the Vision Supplements Review for our tests of vision supplements, including those that most closely resemble the AREDS2 formula and our Top Picks among products.
Clinical Update
10/02/2021
Sanitizer Warning
Consumers are being warned not to use a specific hand-held UV sanitizing wand that could cause skin or eye injury within seconds of use. Details are in our updated article about UV Light Sanitizers and COVID.
Clinical Update
8/03/2019
Concerns With Colloidal Silver
Consuming colloidal silver may affect the eyes and cause a type of vasculitis, according to recently published reports. Get the details in our answer to the question: Is ingesting colloidal silver helpful for any condition and is it safe to use?
Clinical Update
7/03/2020
Lycopene for Wrinkles?
A recent study tested the effects of lycopene supplementation on wrinkles around the eyes ("crow's feet") in older women. Find out if it helped in the What It Does section of the Lycopene Supplements Review. Also see our Top Pick among lycopene supplements.
Clinical Update
3/07/2020
Melatonin and Cataracts
Do you have cataracts (clouded lenses of the eye)? A recent study suggests that having them removed and replaced with a certain type of intraocular lens may increase natural melatonin production. For details, see the ConsumerTips section of the new Melatonin Supplements Review. Also see our Top Picks among melatonin supplements.
Clinical Update
4/28/2020
Early Sign of Thiamin Deficiency
Thiamin (vitamin B1) deficiency can cause lasting neurologic damage and affect heart function. It can occur with poor nutrition and as a result of taking certain diuretics. A recent case highlighted an early sign of thiamin deficiency that involves the eyes. We've added this information to the Thiamin section of the B Vitamin Supplements Review. Also see our Top Pick for thiamin and B complexes that contain thiamin. You can also get thiamin from a good multivitamin.
Clinical Update
9/03/2019
Collagen for Wrinkles?
A new study put a popular branded collagen ingredient to the test, measuring its effects on collagen content in the skin and on facial wrinkles, including wrinkles around the eyes. See the results in our updated answer to the question: Do collagen or hyaluronic acid supplements really help aging or wrinkled skin?
Clinical Update
11/08/2015
When to Use Lutein
Lutein and zeaxanthin can help people who have macular degeneration of the eye. However, a recent study found that taking lutein and zeaxanthin may only help those with existing low levels of these compounds. Get more details, including dosage, in the Lutein and Zeaxanthin Review >>
Clinical Update
8/23/2016
Omega-3s and Diabetic Eye Disease
Getting the omega-3 fatty acids EPA and DHA from eating fish is associated with a much lower risk of developing sight-threatening retinopathy in diabetics, according to a recently published study. The same benefit has been shown in non-diabetics. But do omega-3 supplements help? Find out in the "What It Does" section of the Fish Oil Supplements Review >>
Clinical Update
1/19/2014
Multivitamin Lowers Cataract Risk
A large, long-term study using a low-dose multivitamin found that it reduced the risk of developing cataract (a clouding of the lens of the eye) by 9% compared to placebo. Interestingly, the reduced risk of cataract was negated when a separate vitamin C supplement was also taken. For details about the study and dosage, as well as our tests and comparison of multivitamins, see the updated Multivitamin and Multimineral Supplements Review.
Clinical Update
4/20/2014
Probiotic Helps a Little with Allergy
Among people already taking a daily antihistamine, adding a daily probiotic provided a small, additional benefit -- particularly with ocular (eye) symptoms, according to a new study. For more information about the probiotic used, and our tests of probiotic supplements, see the updated Probiotic Supplements Review >>
Clinical Update
10/01/2017
Does Lutein Help?
Lutein is an antioxidant that helps protect the retina of the eye and can slow the progression of macular degeneration. But does taking lutein help if you already have normal macular density? Two studies have "looked" at this question (including one published this week) and, you can "see" the answer in the "What It Does" section of Vision Supplements (Lutein & Zeaxanthin) Review >>
Clinical Update
2/25/2017
Lutein for Stress?
Supplementing with lutein can help some people protect against macular degeneration of the eye. A new study suggests it may also reduce stress. For details, see the "What It Does" section of the Review of Vision Supplements with Lutein and Zeaxanthin (which includes our tests of products) >>
Clinical Update
4/10/2011
Fish Oil and the Eye
A study of over 30,000 women showed that the risk of developing age-related macular degeneration (AMD) was reduced by up to 42% among those consuming the most fish and highest amounts of omega-3 fatty acids DHA and EPA. Find out the specific types and amounts of fish and the amounts of DHA and EPA associated with the greatest reductions in the risk of AMD. See the Fish Oil Supplements Review for more >>
Clinical Update
3/31/2025
Cocoa for Aging Eyes?
Did taking cocoa extract slow macular degeneration (AMD) in older people? Find out what a recent study showed in the Vision section of our Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review, which includes our Top Picks among supplements, cocoas and mixes, dark chocolates and chips, and cacao nibs.
Also see our Top Picks AREDS formulas to slow the progression of AMD in our Vision Supplements Review.
Clinical Update
9/19/2024
Propolis for Vision?
Propolis is found in some eye formulas, but is there good evidence that is helps with conditions such as glaucoma, cataracts, or retinal damage? Find out in our Vision Supplements Review, which includes our Top Picks among lutein and AREDS formula supplements.
Also find out if propolis has shown benefit for fatty liver disease or arthritis.
Clinical Update
10/21/2024
Pistachio Eye Benefits?
Did eating pistachios, a natural source of lutein, improve macular pigment density in the retinas of people with low density levels (a risk factor for age-related macular degeneration)? Find out what a recent study showed in the Macular Degeneration section of our Vision Supplements Review, which includes our Top Pick lutein and AREDS2 supplements
Also see: Do pistachios provide melatonin?
Clinical Update
4/23/2024
Hair Devices & Safety
Can low-level light therapy (LLLT) hair devices such as Theradome or Capillus cause eye damage? Find out in our article about hair supplements and devices.
Clinical Update
12/20/2022
Collagen for Wrinkles?
How much did collagen supplementation reduce the severity of "crow’s feet" wrinkles around the eyes in a recent study? Find out in the Aging skin and wrinkles section of our Collagen Supplements Review. Also see our Top Picks among collagen supplements for skin and for joints.
Clinical Update
7/19/2022
Quercetin for Seasonal Allergies?
Did quercetin improve nasal and eye symptoms among people with seasonal allergies in recent study? Find out in the Seasonal Allergy section of our Quercetin Supplements Review.
Clinical Update
1/19/2024
Pycnogenol for Hearts and Eyes?
We answered the following questions in our article about Pycnogenol and pine bark extracts:
- Does Pycnogenol help control coronary artery calcification?
- Is Pycnogenol beneficial for glaucoma?
Clinical Update
2/06/2024
Omega-7s for Wrinkles?
Did taking an omega-7 supplement decrease wrinkles around the eyes (“crow’s feet”) in a recent study? Find out in our Fish, Krill, and Algal Oil Supplements Review, which includes our Top Pick for getting omega-7s.
Also see: Do any supplements increase skin elasticity or tighten the skin?
Clinical Update
8/29/2023
Counterfeit Vision Supplement
Counterfeit versions of a major brand of eye vitamin have been sold on Amazon. For details, see the Update in our Vision Supplements Review, which includes our Top Picks among vision supplements.
Also see: How to Avoid Buying Counterfeit Vitamins and Supplements
Recalls & Warnings
September 26, 2012
Maker of Eye Health Supplements Warned for Drug Claims
On September 18, 2012, the FDA issued a warning letter to EyeScience Labs, L.L.C. because statements made about the company's Macular Health Formula, Dry Eye Formula and Diabetic Vision Formula supplements were found to constitute drug claims.
Recalls & Warnings
September 08, 2025
FDA Warns Two Sellers of Homeopathic Eye Drops
On August 25, 2025, the FDA issued Warning Letters to Supply Center USA and Homeopathic Educational Services (dba Homeopathic Family Medicine) following reviews of the companies’ websites, which found statements about certain "homeopathic" eye drop products to be drug claims.
Recalls & Warnings
November 29, 2023
Dr. Berne’s Whole Health Products Warned for Contaminated Eye Drop Products, Drug Claims
On November 22, 2023, the FDA issued a Warning Letter to Dr. Berne’s Whole Health Products following review of the company’s website, which found statements about the company’s Dr. Berne’s MSM Eye Drops 5%, Dr. Berne’s MSM Eye Drops 15%, and Dr.
Recalls & Warnings
November 09, 2023
Zazzee Naturals Warned for MSM Eye Drop Claims
On October 30, 2023, the FDA issued a Warning Letter to Dexterity Health, LLC DBA Zazzee Naturals following review of the company’s Amazon storefront, which found statements about the company’s Liquid MSM Drops to be drug claims.
Recalls & Warnings
February 28, 2024
Microbial Contamination Found in LightEyez MSM Eye Drops
On February 15, 2024, the FDA issued a Warning Letter to LightEyez Limited after testing by the agency found the company’s LightEyez.com MSM Eye Drops V2.0 — Eye Repair (MSM Eye Repair Drops) to have microbial contamination in samples of the drops.
Recalls & Warnings
December 07, 2023
Belmont Eyecare Warned for Colloidal Silver, MSM, and Castor Oil Eye Drop Claims
On December 1, 2023, the FDA issued a Warning Letter to Belmont Eyecare LLC, following a review of the company’s website, which found statements about the company’s Colloidal Silver Eye Drops, MSM Eye Drops, Organic Daytime Oil Eye Drops, Organic Daytime Oil Eye Drops Small, Organic ...
News Release
February 16, 2011
Some turmeric and curcumin supplements fail quality review -- ConsumerLab.com finds 20% of turmeric supplements selected for review deliver less than 15% of promised compounds
WHITE PLAINS, NEW YORK — FEBRUARY 16, 2011 — Supplements containing the herb turmeric or its key compound, curcumin, have become popular in the U.S. ConsumerLab.com cautioned today that two out of ten turmeric products recently selected for quality testing were found to provide only 7.
Recalls & Warnings
August 28, 2023
Dr. Berne’s MSM and Castor Oil Eye Drops Recalled Due to Bacterial and Fungal Contamination
On August 26, 2023, Dr. Berne’s Whole Health Products issued a recall of all lots of its MSM DROPS 5%, 15% Solution, Dr. Berne’s Organic Castor Oil Eye Drops and Dr. Berne’s MSM MIST 15% Solution due to the risk of bacterial and fungal contamination.
Recalls & Warnings
September 14, 2023
FDA Warns CVS, Walgreens, Similasan & Others for Eye Drop Violations
On September 11, 2023, the FDA issued Warning Letters to the following eight sellers of homeopathic and other types of eye drops regarding the products noted in italics due to a variety of violations of FDA regulations, most notably that they were marked with claims suggesting that they could cure, ...
News Release
December 16, 2009
"Eye health" supplements vary widely in formula but product quality is high according to ConsumerLab.com
"Eye Health" Supplements Vary Widely in Formula But Product Quality is High According to ConsumerLab.com
News Release
December 09, 2008
Tests of zinc supplements by ConsumerLab.com show only some provide dosage proven to shorten colds, reduce eye disease. Lead contamination found in one product.
WHITE PLAINS, NEW YORK — DECEMBER 9, 2008 — Zinc supplements can shorten colds and reduce the progression of advanced macular degeneration, among other uses. But a new report by ConsumerLab.
News Release
October 28, 2008
Tests reveal quality problems with vitamin A supplements -- New ConsumerLab.com report focuses on vitamin A, including beta-carotene and cod liver oil
WHITE PLAINS, NEW YORK — OCTOBER 28, 2008 — ConsumerLab.com is cautioning that the amount vitamin A in some supplements may be much higher or lower than the amount stated on labels. Among ten supplements recently selected for testing, ConsumerLab.
News Release
July 12, 2007
ConsumerLab.com finds lutein & zeaxanthin supplements vary widely in strength but most meet ingredient claims — Right amount of antioxidant may help prevent macular degeneration and improve vision
WHITE PLAINS, NEW YORK — July 12, 2007 — ConsumerLab.com announced today that all but one of the thirteen lutein and zeaxanthin supplements it recently chose to test met quality standards.
News Release
June 12, 2007
Tests show many milk thistle supplements low in key component — Seven products fail ConsumerLab.com testing of herb used in diabetes and liver diseases
WHITE PLAINS, NEW YORK — June 12, 2007 — Tests by ConsumerLab.com of nine milk thistle supplements that it selected showed that only two contained the expected amounts of silymarin compounds, believed to be the active constituent of milk thistle.
News Release
May 15, 2007
ConsumerLab.com finds some alpha lipoic acid supplements come up short — Improper formulation may cause ingredient degradation
WHITE PLAINS, NEW YORK — May 15, 2007 — ConsumerLab.com announced that two of eleven alpha lipoic acid supplements that it recently chose to test contained significantly less ingredient than promised on their labels.
News Release
April 24, 2007
ConsumerLab.com reports improvement in quality of SAM-e supplements used for osteoarthritis and depression — Recent testing shows quality is up since studies in 2000 and 2003
WHITE PLAINS, NEW YORK — April 24, 2007 — ConsumerLab.com announced that all eight SAMe supplements that it recently selected for review passed quality testing — an improvement over tests in previous years. SAMe (S-adenosyl-methionine) is used to treat depression and joint pain.
Recalls & Warnings
February 06, 2023
Recalled Eye Drops Linked to Vision Loss and One Death
On February 2, 2023, Global Pharma Healthcare issued a nationwide recall of its Artificial Tears Lubricant Eye Drops due to potential contamination with bacteria that can cause serious infection, blindness, and death.
Recalls & Warnings
July 19, 2024
Walnuts Recall Due to Listeria
On July 19, 2024, Stutz Packing Company recalled its 1-pound packages of Shelled Walnuts due to potential contamination of Listeria monocytogenes. Routine sampling realized that the finished products contained bacteria. Currently, there have been no reports of illness related to this call.
News Release
April 11, 2007
Many arthritis supplements lack key listed ingredient — ConsumerLab.com finds chondroitin missing or low in eight supplements; Glucosamine and MSM also tested
WHITE PLAINS, NEW YORK — April 11, 2007 — Forty percent of the supplements for osteoarthritis that ConsumerLab.com recently selected for testing were found to lack all or some of their key ingredients. Osteoarthritis is the most common type of arthritis, affecting an estimated 20.
Recalls & Warnings
July 22, 2022
FDA Warns Seller of Vision, Calcium & Magnesium Supplements and More
On June 14, 2022, the FDA issued a warning letter to New Sun Inc. following inspection of the company’s website which found statements about the company’s products to be drug claims.
Recalls & Warnings
August 28, 2024
Now Foods Recalls Raw Brazil Nuts Due to Mold and Yeast
On August 14, 2024, NOW Foods issued a recall of its Now Foods Whole & Unsalted Raw Brazil Nuts because they were found to be contaminated with mold and yeast.
Recalls & Warnings
August 25, 2022
Oregon’s Wild Harvest Warned by FDA for Manufacturing Violations
On July 8, 2022, the FDA issued a warning letter to Oregon’s Wild Harvest, Inc.
Recalls & Warnings
June 12, 2023
Children's Liquid Vitamin Supplement Recalled
On June 7, 2023, Procaps S.A. de C.V. issued a voluntary recall of various lots of Laboratorios Lopez’s Bacaolinita 8 fl. oz children's liquid vitamin supplement because it contains undeclared PEG-40 hydrogenated castor oil.
Recalls & Warnings
April 03, 2024
FDA Warns Ambaya Gold for Promoting Products for Depression, Cancer, & Arthritis
On December 5, 2023, the FDA issued a Warning Letter to Ambaya Gold Health Products, LLC following review of the company’s website and social media, which found statements about the company’s Brain Balance, Immune System Boost, Dentist In A Bottle, Essensiac, Fulvic Green, Silver ...
Recalls & Warnings
May 01, 2024
Organic Walnuts Recalled Due to E. Coli Risk
On April 30, 2024, Gibson Farms recalled two lots of Organic Light Halves and Pieces shelled walnuts due to potential contamination with E. coli (Escherichia coli).
News Release
February 14, 2007
ConsumerLab.com reports on anti-depressant herb — St. John's Wort — Few supplements pass quality testing
WHITE PLAINS, NEW YORK — FEBRUARY 14, 2007 — St. John's wort may be helpful in treating mild to moderate cases of clinical depression, but a new report from ConsumerLab.com shows that few of the herbal products tested met quality standards. Americans purchased $64 million of St.
News Release
January 19, 2007
Consumers warned of problems with multivitamins — ConsumerLab.com uncovers defects in over half of products tested
WHITE PLAINS, NEW YORK — JANUARY 19, 2007 — Fifty-two percent of multivitamins recently selected for testing by ConsumerLab.com were found to be contaminated with lead, unable properly break apart, or to contain significantly more or less ingredient than claimed.
Recalls & Warnings
February 17, 2010
FTC Warns of Deceptive Claims with Children's Omega-3 Supplements
On February 17, 2010, the Federal Trade Commission (FTC) announced that it had sent letters to 11 companies that promote various omega-3 fatty acid supplements, telling them they should review their product packaging and labeling to make sure they do not violate federal law by making baseless ...
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
March 17, 2015
Seller of Tea Warned for Drug Claims
On March 4, 2015, the FDA issued a warning letter to Four Elements Organic Herbals, LLC because statements made about HERBAL TEA Power, Energy & Stamina, ORGANIC HERBAL TEA Tulsi TelepaTea, ORGANIC HERBAL TEA To Your Health, Hawthorn Fresh Herb Extract, Elderberry Fresh Herb ...
Recalls & Warnings
March 06, 2015
Seller of Tea Drinks Warned for Drug Claims
On February 26, 2015, the FDA issued a warning letter to the seller of Anamu CancerHerb Tea Box, Anamu CancerHerb Tea Box with Honey and Organic Bottled Tea Drinks because statements made about these products on the websites, www.cancerherbtea.com and www.anamucancerherbtea.
Recalls & Warnings
February 17, 2015
Seller of Muscle Supplements Containing Synthetic Steroids Warned
On February 9, 2015, the FDA issued a warning letter to A2Z Industries, LLC, following a facility inspection which found the company's muscle enhancing supplements, including HaloV and EPI2A3A to be labeled as containing synthetic steroids.
Recalls & Warnings
February 06, 2015
Seller of Aloe Supplement Warned for Drug Claims
On January 21, 2015, the FDA issued a warning letter to Pristine Nutraceuticals, LLC, following a review of the company's website, http://www.digestaqure.com, which found statements made about DigestaCure to be drug claims.
Recalls & Warnings
June 30, 2022
FDA Warns Seller of Vision and Allergy Supplements
On May 26, 2022, the FDA issued a warning letter to Golden Lab LLC following an inspection of the website, which found statements about the company’s DoctoRx’s Optimal Formula Ocular Pressure & Optic Nerve Support Formula Ocular Health Capsule, DoctoRx’s Optimal Formula ...
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
News Release
December 12, 2006
Probiotic supplements grow in popularity but viable bacteria missing in many — ConsumerLab.com cautions consumers to select probiotics carefully and store them properly
WHITE PLAINS, NEW YORK — DECEMBER 12, 2006 — Sales of supplements containing beneficial organisms (probiotics) have grown rapidly, but a new test report from ConsumerLab.com shows that 44% contained fewer viable organisms than claimed or generally known to be effective.
News Release
November 13, 2006
Tests of "muscle" supplements finds some "weak" products but most contain expected creatine, HMB, or amino acids — Review of muscular enhancement supplements published by ConsumerLab.com
WESTCHESTER, NEW YORK — MONDAY, NOVEMBER 13, 2006 — Bodybuilders and athletes often turn to supplements to enhance muscle size and strength.
News Release
August 25, 2004
ConsumerLab.com finds not all vitamin E products meet claims — Results for 33 vitamin E supplements and skin products released today
WHITE PLAINS, NY — Wednesday, August 25, 2004 (Updated 10/24/04) — ConsumerLab.com announced today that five vitamin E products failed to pass recent testing for having either too little vitamin E and/or for containing synthetic vitamin E when claiming to be natural.
News Release
January 29, 2004
ConsumerLab.com finds lutein supplements vary widely in strength but most meet ingredient claims — Right amount of antioxidant compound may reduce risk of blindness
WHITE PLAINS, NY — January 29, 2004 — ConsumerLab.com announced today that only one of the 19 lutein/zeaxanthin supplements it recently tested failed to contain the claimed ingredients. Although product quality was generally high, ConsumerLab.
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
February 09, 2022
FDA Warns Seller of Colloidal Silver Eye Drops, Copper Products & More
On February 1, 2022, the FDA issued a warning letter to New Earth Healing Essentials, LLC d/b/a 5D Full Disclosure following a review of the company’s website, which found statements made about some of the company's products, including Plasma Colloidal Silver Eyedrops, Gaia’s ...
Recalls & Warnings
March 11, 2024
Healthex Warned for Manufacturing Violations
On June 15, 2023, the FDA issued a Warning Letter to Healthex Distributors, Inc.
News Release
August 28, 2003
Some problems persist with ginseng supplements but overall quality improves according to ConsumerLab.com — Test results published online today
WHITE PLAINS, NY — August 28, 2003 — In contrast to its testing three years ago in which nearly 60% of Asian and American Ginseng supplements were found to have significant problems, testing of recently purchased products by ConsumerLab.
Recalls & Warnings
March 09, 2022
FDA Warns Seller of Magnesium, CBD, Herbal Extracts & More
On February 9, 2022, the FDA issued a warning letter to Bea Lydecker’s Naturals, Inc.
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
November 17, 2021
Mountain Meadow Herbs "Cleanse" Recalled, Risk of Injury from Exploding Bottle
On November 17, 2021, Mountain Meadow Herbs issued a recall of 54 bottles of Mountain Meadow Herbs brand Candida Flush due to the bottles becoming pressurized over time in storage.
Recalls & Warnings
August 07, 2023
Aerosol Bed Bug & Flea Killer Recalled Due to Injury Risk
On August 3, 2023, Maggie’s Farm issued a recall of the company’s Aerosol Bed Bug & Flea Killer products because the aerosol can can rupture and expel shrapnel, posing an injury and laceration hazard to consumers.
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
March 18, 2021
Don't Drink Real Water Alkaline Water, FDA Warns After Reports of Liver Illness
On March 16, 2021, the FDA warned consumers not to drink or use Real Water bottled alkaline water while it investigates reports of hepatitis associated with the product.
Recalls & Warnings
October 27, 2004
Some Supplements Can Damage Eyes At High Doses and with Topical Use
As reported by Reuters Health on October 21, 2004, an article in the current issue of the American Journal of Ophthalmology suggests that some herbal remedies and nutritional supplements can damage the eyes.
Recalls & Warnings
July 21, 2022
UV Light Wands That May Cause Injury, According to the FDA
The FDA recently warned consumers of potential exposure to unsafe levels of ultraviolet-C (UV-C) radiation associated with the use of certain brands of ultraviolet (UV) wands, as found from testing conducted by the agency.
Recalls & Warnings
February 06, 2023
FDA Warns Companies Promoting Products to Treat Monkeypox, COVID-19, & Other Viruses
On January 30, 2023, the FDA issued a warning letter to four companies following review of the company websites, which found statements about company products to be drug claims because they were promoted to mitigate, prevent, treat, diagnose, or cure viruses such as monkeypox.
Recalls & Warnings
September 21, 2023
Moor Herbs, Inc Warned for Manufacturing Violations, Drug Claims
On August 1, 2023, the FDA issued a Warning Letter to Moor Herbs, Inc.
Recalls & Warnings
May 15, 2016
Seller of Vision Supplements Warned for Drug Claims
On April 28, 2016, the FDA issued a warning letter to Macular Health, LLC.
Recalls & Warnings
March 10, 2015
Seller of Vision Supplements Warned for Manufacturing Violations, Drug Claims
On February 26, 2015, the FDA issued a warning letter to Biosyntrx, following a facility inspection which found the company's products, including BioTears Oral Gel Caps, ZoOmega-3 Concentrated Pharmaceutical-Grade Fish Oil, EpiCor A Nutrient-dense High Metabolite Immunogen, Sight C+ Mineral ...
Recalls & Warnings
February 19, 2005
Seller of Eye Supplement Purporting to Restore Vision and Eliminate "Floaters" Settles FTC Charges
On February 15, 2005, the Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, have settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular ...
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
Recalls & Warnings
May 20, 2005
Seller of "Ocular Nutrition" Supplement Settles FTC Charges
On March 16, 2005, The Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular Nutrition) – ...
Recalls & Warnings
July 28, 2010
Choking Concern Prompts Eye Supplement Recall
On July 28, 2010, the U.S. FDA posted a recall by Bausch + Lomb of its PreserVision® Eye Vitamin AREDS 2 Formula with Omega 3 soft gels, only available within the United States.
Recalls & Warnings
January 04, 2020
Hormonal Balance Supplement Tied to Liver Failure
An otherwise healthy 23-year old woman in Texas is reported to have recently developed liver failure (requiring a liver transplant) after taking the supplement Balance (from Alani Nu) for four months.
Recalls & Warnings
April 30, 2021
FDA Warns Seller of Fish Oil, Apple Cider Vinegar, Collagen, and Elderberry Supplements
On April 13, 2021, the FDA issued a warning letter to Orphic Nutrition following a review of the company's websites, which found statements made about the products Apple Cider Vinegar Capsules, Ashwagandha Capsules, Garcinia Cambogia Capsules, Premium Collagen Peptides Capsules, Elderberry ...
Recalls & Warnings
February 11, 2012
Herbal Sprays for Colds and Cold Sores Recalled
On February 10, 2012 the U.S. FDA posted a notice announcing the recall of all "Koff & Kold" and "Kold Sore" sprays by Wholistic Herbs Inc. The products were distributed throughout the United States.
Recalls & Warnings
December 22, 2005
Health Canada Warns Consumers Not to Take Chaparral
On December 21, 2005, Health Canada (Canada's health ministry) warned consumers not to ingest the herb chaparral in the form of loose leaves, teas, capsules or bulk herbal products because of the risk of liver and kidney problems.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
November 21, 2017
Health Canada Calls for Stronger Warnings of Liver Risk on Green Tea Extract Products
On November 15, 2017, Health Canada (the Canadian equivalent of the FDA) recommended stronger warning statements about the risk of liver toxicity on labels of supplements containing green tea extract sold in Canada.
Recalls & Warnings
January 02, 2004
Canada Reiterates Warning on Products Containing Kava
On December 23, 2003, Health Canada reminded Canadian consumers of the serious risks associated with the use of products containing kava.
Recalls & Warnings
May 01, 2009
FDA Warns Consumers to Stop Using Hydroxycut -- Product Being Tested by ConsumerLab.com
On May 1, 2009, the U.S. Food and Drug Administration warned consumers to immediately stop using Hydroxycut products by Iovate Health Sciences Inc., of Oakville, Ontario and distributed by Iovate Health Sciences USA Inc. of Blasdell, N.Y.
Recalls & Warnings
March 28, 2011
Dangerously High Levels of Vitamins A and D in Product Prompt FDA Warning
On March 28, the U.S. FDA warned consumers to stop using Soladek, a vitamin-solution product marketed by Indo Pharma, S.A., of the Dominican Republic, because the product may contain dangerously high levels of vitamins A and D.
Recalls & Warnings
January 07, 2002
Liver Toxicity with Kava
As announced in a December 19, 2001 letter to healthcare professionals, The Food and Drug Administration (FDA) is investigating whether the use of dietary supplements containing kava (also known as kava kava or Piper methysticum) is associated with liver toxicity.
Recalls & Warnings
January 27, 2015
Seller of Aloe, Sexual Enhancement Supplements and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Aloe Man, Inc., following a facility inspection which found the company's products, including The Aloe Man's Super Bright, Dr. Johnson's Maximum Desire, Dr. Johnson's Body Healer, and Dr.
Recalls & Warnings
July 29, 2009
FDA Warns Against Body Building Supplements with Steroid-like Compounds
On July 28, 2009, the U.S. FDA notified the public about new safety information concerning products marketed for body building and increasing muscle mass.
Recalls & Warnings
January 02, 2004
Health Canada Warns Consumers Not to Use Health Products Containing Comfrey
On December 12, Health Canada advised Canadian consumers not to ingest the herb comfrey or any health products that contain comfrey because they might contain a compound called echimidine, which may cause liver damage.
Recalls & Warnings
August 25, 2002
Canadian Recall and Stop-Sale Order on All Kava Products
Health Canada is issuing a stop-sale order for all products containing the herb kava after a safety assessment concluded there is insufficient evidence to support their safe use. The department is also requesting the recall of these products from all levels of the market.
Recalls & Warnings
January 29, 2004
U.S. Action on Mad Cow -- Supplements Included As Well as Food
On Jan. 26, 2004, the U.
Recalls & Warnings
February 20, 2013
Cold, Flu and Stress Supplement Company Warned For Adulteration, Drug Claims And More
On October 5, 2012, the FDA issued a warning letter to dietary supplement manufacturer Wholistic Herbs, Inc. following a facility inspection which found the company's products, including At-Ease, Morning Calm, Aller-Ban, Stomach Flu, Kold & Koff, Kold Sore, and L.
Recalls & Warnings
February 21, 2013
Pet Supplement Company Issues Recall Due To Potential Bacterial Contamination
On February 20, 2013, animal supplement company Nutri-Vet, LLC issued a recall of Nutri-Vet and NutriPet Chicken Jerky Products because they may be contaminated with Salmonella.
Recalls & Warnings
October 09, 2013
FDA Warns Consumers Not to Use OxyElite Pro As It Investigates Link With Hepatitis
On October 8, 2013, the FDA warned consumers not to buy or use USPLabs’ weight loss supplement OxyElite Pro because it has been linked with 24 cases of acute non-viral hepatitis in Hawaii.
Recalls & Warnings
August 27, 2013
FTC Refunds Consumers of Children's Vitamins as Part of Deceptive Advertising Settlement
On August 8, 2013, the Federal Trade Commission (FTC) announced it has mailed 10,144 refund checks to consumers who purchased Disney or Marvel Hero -themed children's vitamins, including Disney Princesses, Winnie the Pooh, Finding Nemo, and Spider-Man varieties, between May 1, 2008 and September ...
Recalls & Warnings
June 05, 2018
FDA Warns Seller of Colloidal Silver
On May 17, 2018, the FDA issued a warning letter to Silver Armor, Inc.
Recalls & Warnings
November 04, 2016
Energy Drink Linked to Case of Acute Hepatitis
A case of acute hepatitis has been linked to daily consumption of energy drinks, according to a report published on November 1, 2016, in BMJ Case Reports.
Recalls & Warnings
March 25, 2002
FDA Advises Consumers of Risk of Severe Liver Toxicity with Kava
On March 25, 2002 the U.S. Food and Drug Administration (FDA)issued a Consumer Advisory, as well as a letter to health care providers, advising of the potential risk of severe liver injury associated with the use of kava-containing dietary supplements.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
February 15, 2006
New Zealand Warns of Liver Toxicity from Black Cohosh
On February 9, 2006, New Zealand's Therapeutic Goods Administration (TGA) announced new labelling and consumer information for medicines containing Black cohosh (Cimicifuga racemosa).
Recalls & Warnings
September 17, 2003
FDA Warns of Illness from Star Anise Teas
On September 10, 2003, the Food and Drug Administration (FDA) advised consumers not to consume "teas" brewed from star anise.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
November 20, 2013
OxyElite Pro Recall Expanded
On November 19, 2013, USPlabs LLC expanded its recall of OxyElite Pro products to include Raspberry Lemonade OxyELITE Pro Super Thermo Powder (4.6 oz., UPC #094922447494).
Recalls & Warnings
August 13, 2016
Seller of Omega-3, Ginkgo, Vision Supplements & More Warned for Drug Claims
On July 22, 2016, the FDA issued a warning letter to Viva Nutraceuticals (J & E International Corporation) following a review of the company's website and promotional literature, which found statement made about its products, Eyestonia, Ginkgo Biloba 60mg, Golden Omega-3 Max, ...
Recalls & Warnings
February 09, 2016
St. John's Wort Supplements Recalled
On February 8, 2016, the Medicines and Healthcare products Regulatory Agency (MHRA) (the British equivalent of the FDA) announced that certain St. John's wort supplements are being recalled because they contain excessive levels of a toxic pyrrolizidine alkaloid (PA).
Recalls & Warnings
July 12, 2015
Marketers of Memory Supplement to Pay $1.4 Million to Settle FTC Charges
On July 8, 2015, the FTC announced the marketers of Procera AVH (Brain Research Labs Inc.) have agreed to pay $1.4 million to settle charges they made deceptive claims that the supplement could significantly improve memory, mood and cognitive function.
Recalls & Warnings
May 22, 2018
FDA Warns Companies Selling "Sun Protection" Supplements
On May 18, 2018, the FDA issued warning letters to four companies selling supplements for sun protection because statements made on product labels, websites and in marketing materials were found to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
December 23, 2017
Seller of Meal Replacement, Protein Drinks, Cranberry and More Warned for Manufacturing Violations
On July 6, 2017, the FDA issued a warning letter to Professional Botanicals, Inc.
Recalls & Warnings
December 20, 2017
Seller of Supplements for Pain and Allergies Warned for Manufacturing Violations, Drug Claims
On December 13, 2017, the FDA issued a warning letter to GnuPharma Corporation, following a facility inspection which found a number of the company's products, including Relief capsules, Foundation capsules, Aller-geez capsules, Fit capsules, Aller-Geez Tea, Foundation herbal tea and ...
Recalls & Warnings
June 22, 2010
Pet Vitamin Recalled for Salmonella Risk
On June 22, 2010, the FDA reported that United Pet Group, Cincinnati, Ohio is voluntarily recalling all unexpired lots of its PRO-PET ADULT DAILY VITAMIN Supplement tablets for Dogs due to possible Salmonella contamination. This product is not among those included in ConsumerLab.
Recalls & Warnings
May 03, 2013
Homeopathic Company Warned For Drug Claims and Improper Labeling
On March 12, 2013, the FDA issued a warning letter to Natural Medicine Associates, Inc.
Recalls & Warnings
July 18, 2010
Expansion of Pet Supplement Recall for Salmonella Risk; Many Brands Affected
On July 2, 2010, the U.S.
Recalls & Warnings
November 11, 2013
Workout Supplement OxyElite Pro Recalled After Being Linked to Serious Liver Illness
On November 10, 2013, the FDA announced that USPLabs LLC is recalling workout supplements OxyElite Pro Super Thermo capsules, OxyElite Pro Ultra-Intense Thermo capsules and OxyElite Pro Super Thermo Powder because they have been linked to a number of cases of acute non-viral hepatitis, one of which ...
Recalls & Warnings
April 16, 2014
Seller of Aloe, Thyroid and Other Supplements Warned for Manufacturing Violations and Drug Claims
On March 21, 2014, the FDA issued a warning letter to Aloe Man International Corp.
Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.