Product Reviews and Information for Diabetes
Search term may appear only in full report available to members. Join now for full access.
CL Answer
Which supplements and foods can help lower or control blood sugar?
Find out which supplements can help lower or control blood sugar. Supplements including turmeric/curcumin, fiber, cinnamon, and ginseng are believed to help lower blood sugar.
Product Review
Vitamin D Supplements Review (Including Calcium, Magnesium, Vitamin K, and Boron)
Find the Best Vitamin D Supplement and Avoid Problems

Product Review
Cinnamon Supplements and Spices Review
CAUTION: Limit Use of Some Cinnamon Due to Liver Toxin. See Our Findings and Top Picks.

Product Review
Extra Virgin Olive Oil Review
Many Extra Virgin Olive Oils Don't Seem to Make the Grade

Product Review
Turmeric and Curcumin Supplements and Spices Review
See Our Top Picks Among Turmeric Products

Product Review
Apple Cider Vinegar Review — Bottled Liquids and Supplements
Find the Best Apple Cider Vinegar. See Which Apple Cider Vinegar Liquids and Supplements Passed Our Tests of Quality.

CL Answer
I have type 2 diabetes. Should I be concerned about ingredients like maltodextrin and maltitol that are found in some protein bars and drinks?
Learn more about maltitol and maltodextrin and their effects on blood glucose levels for people with type 2 diabetes.

Product Review
B Vitamin Supplements Review (B Complexes, B6, B12, Biotin, Folate, Niacin, Riboflavin & More)
See Our Top Picks and Which 5 Failed Testing

Product Review
Milk Thistle Supplements Review
2,800% Difference Found Among Milk Thistle Supplements. Find the Best Milk Thistle Based on Our Tests and See Our Top Picks.

CL Answer
Sugar Substitutes: Pros, Cons, and Best Choices
Learn about the pros and cons of using sugar substitutes and artificial sweeteners in place of table sugar. Sweeteners discussed include stevia, monk fruit, and other high-intensity sweeteners; xylitol, erythritol, allulose and other low-calorie sweeteners; and agave syrup, black sugar, coconut sugar, honey, molasses and other sugar alternatives.

Product Review
Protein Powders, Shakes, and Meal Replacements Review
Find Out Which Protein Products Passed or Failed Our Tests

Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

Product Review
Chromium Supplements Review
Chromium Supplements Review. Best Chromium Supplements Identified -- By Strength.

Product Review
Berberine and Goldenseal Supplements Review
Tests Reveal the Best and Worst Berberine and Goldenseal Products

Product Review
Probiotic Supplements Review (Including Pet Probiotics)
See What's Really in Probiotics and Our Top Picks

CL Answer
Does Gynostemma pentaphyllum really work as an AMPK activator and help for diabetes, high cholesterol, or weight loss?
Find out if Gynostemma pentaphyllum ( jiaogulan) works as an AMPK activator and helps to lower blood sugar and treat diabetes. ConsumerLab.com's answer explains.

Product Review
Alpha-Lipoic Acid Supplements Review
Choose the Best Alpha-Lipoic Acid Supplement — See the Amounts of Active "R-Isomer" We Found

Product Review
Amla Supplements Review
Only 3 of 8 Amla Powders and Pills Pass CL's Tests

Product Review
Magnesium Supplements Review (Including Calcium, Vitamins D & K, and Boron)
Find Out What Magnesium Does, Who Needs It, and Our Top Picks Among Supplements
Product Review
Ginseng Supplements Review
Find the Best Ginseng Supplement. Key "Ginsenosides" Found to Range 10-Fold Across Products.

CL Answer
Akkermansia muciniphila: Health Benefits and Safety
Akkermansia muciniphila has gained attention for its potential benefits in managing insulin resistance, diabetes, weight loss, cancer, and other health conditions, but does it really work, and is it safe? Find out.

Product Review
Multivitamin and Multimineral Supplements Review
Best Multivitamins -- Caution with Gummies

Product Review
Resveratrol Supplements Review (From Red Wine, Knotweed, and Other Sources)
See Which Resveratrol Supplements Were Best In Our Tests and Comparisons. Learn What Resveratrol Can and Can't Do.

Product Review
Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review -- Sources of Flavanols
Is Your Chocolate or Cocoa Healthful or Toxic? Find the Best Dark Chocolate, Cocoa Powder and Cocoa Supplements Based On Our Tests.

Product Review
Holy Basil Supplements Review
Find the Best Holy Basil (Tulsi) Supplements. Tests and Reviews of Popular Holy Basil Supplements & CL's Top Pick.

Product Review
Coconut Waters Review
Best Coconut Water? See Our Tests and Comparisons of Popular Brands Looking at Sugar, Electrolytes, Taste and Price.

Product Review
Olive Leaf Extract Supplements Review
Learn What Olive Leaf Does and the Best Brands

Product Review
Collagen Supplements Review
See our Top Picks for Wrinkles and Joints

Product Review
Glutathione Supplements Review
Learn How Glutathione Products Differ, Which Failed, and Our Top Picks

Product Review
Bilberry Supplements Review
Choose the Best Bilberry Supplement. Some Bilberry Is Not Authentic!

Clinical Update
5/26/2023
Sweeteners & Diabetes
Which sweeteners are most suitable for people with diabetes? Find out in the Diabetes section of our article about sweeteners.
Product Review
Fruits, Veggies, and Other Greens Supplements Review (Including Spirulina and Chlorella)
Avoid Lead in Greens, Problems with Pills, and Don't Give Up Eating Whole Foods.

Product Review
Selenium Supplements Review
Choose the Best Selenium Supplement. Find Out If You Need Selenium and Which Supplement Is Our Top Pick.
CL Answer
Do any supplements, like Nervive, help with nerve pain, like sciatica, diabetic neuropathy or postherpetic neuralgia?
Find out which supplements for nerve pain, including fish oil and alpha-lipoic acid, are effective for sciatica, diabetic neuropathy, or postherpetic neuralgia.

CL Answer
Are L-carnosine supplements helpful and safe?
Learn about the potential health benefits of L-carnosine, as well as possible safety concerns, drug interactions and cost.

Product Review
CoQ10 and Ubiquinol Supplements Review
Find the Best CoQ10 and Ubiquinol Supplements and Learn How They Differ

CL Answer
I read that statins can cause diabetes. Is this true also with red yeast rice?
Find out if red yeast rice supplements increase the risk of diabetes, as some statin medications do. ConsumerLab.com's answer explains.

CL Answer
Are Moringa Leaf Supplements Helpful and Safe?
Are moringa leaf supplements beneficial for diabetes, lowering cholesterol, lowering blood pressure, improving liver function, and other conditions, and are they safe? Find out.

Product Review
Red Yeast Rice Supplements Review
50% of Red Yeast Rice Supplements "NOT APPROVED" in CL Tests

Product Review
Digestive Enzyme Supplements Review
Big Differences in Strength Found Among Digestive Enzyme Supplements. CL Tests Reveal Which Are Best.

Product Review
Canned Tuna, Salmon, Sardines & Mackerel Review
Find Fish With the Least Mercury & Arsenic and Most Omega-3s

Product Review
Ginger Supplements, Chews & Spices Review
Tests Reveal Best and Worst Ginger Supplements & Spices. Poor Quality and Lead Contamination Discovered in Some Products.

Product Review
Green Tea Review: Tea Bags, Loose Leaf Tea, Matcha Powders, and Supplements
Some green teas provide barely any green tea polyphenols, while some others are high strength. See the Test Results and Our Top Picks for Green Tea.

CL Answer
What are the health benefits of green tea, and is it safe?
ConsumerLab explains green tea's potential health benefits for everything from fighting cancer to helping your heart.

Product Review
Manuka Honey Review
Find the Best Manuka Honey Based on Quality, Taste, and Value. See our Top Picks.

CL Answer
Is the theobromine in chocolate and cocoa good or bad for me?
Find out the benefits and risks of theobromine -- and how much is in dark chocolate.

Clinical Update
4/01/2018
Type 2 Diabetes and Vitamin D
Maintaining an adequate level of vitamin D is associated with a lower risk of type 2 diabetes. But does giving vitamin D to people with existing type 2 diabetes help them with glucose control? Find out what research is showing in the What It Does section of the Vitamin D Supplements Review. (Also see our Top Picks among vitamin D supplements.)
Clinical Update
6/09/2019
Vitamin D for Diabetes Prevention
Does high-dose vitamin D help prevent people with pre-diabetes from progressing to type 2 diabetes? See the results of a major new study in the What It Does section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
8/03/2019
Vitamin D & Diabetes
Vitamin D supplementation can improve insulin sensitivity in some people at risk for type 2 diabetes according to a recent clinical study. For details about this, as well as related research, see the Diabetes, insulin resistance and glucose control section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
12/02/2012
Vitamin D and Diabetes Risk
A new study shows a strong association between lower levels of vitamin D in the blood and a higher risk of developing type 1 diabetes (i.e., requiring insulin). The risk of diabetes was more than 3.5 times as great among people with low levels of vitamin D compared to those with higher levels -- although no additional benefit was found for people with the highest levels of vitamin D. Find out more -- including the specific level associated with the lowest risk of type 1 diabetes -- in the updated Vitamin D Supplements Review, which includes our test results for 51 vitamin D supplements. More >>
Clinical Update
8/02/2011
Vitamin D and Diabetes Risk
A recent report showed that the risk of developing type 2 diabetes increases when vitamin D intake is low. The increased risk of diabetes is even more dramatic when vitamin D in the blood is below adequate levels. Get the details in the update to the Vitamin D Supplements Review >>.
Clinical Update
4/01/2015
Vitamin D for Diabetic Neuropathy
Supplementing with vitamin D can reduce pain and symptoms of diabetes-related peripheral neuropathy in people with type 2 diabetes who are vitamin D deficient, a recent study reports. More about the use of vitamin D in diabetes and many other conditions, as well as our product test results, are found in the Vitamin D Supplements Review >>
Clinical Update
5/30/2017
Olive Oil Helps in Pre-Diabetes
Adding extra virgin olive oil to a meal decreased the rise in blood sugar and increased insulin levels after the meal, according to a new study in people with pre-diabetes. Similar benefits with olive oil have been shown in people with type 1 and type 2 diabetes. For details, see the "Insulin Control and Blood Sugar" section of the Extra Virgin Olive Oils Review, which includes our tests and comparisons of popular olive oils on the market.
Clinical Update
10/24/2023
Does Cocoa Lower Diabetes Risk?
Did supplementing with cocoa flavanols reduce the risk of developing type 2 diabetes? Find out what a large study found in the Blood Sugar, Insulin Resistance and Diabetes section of our Dark Chocolate and Cocoa Supplements Review, which includes our Top Picks for cocoa and dark chocolate.
Also see our article about supplements and food for blood sugar control.
Clinical Update
8/08/2024
Milk Thistle for Diabetes?
Does taking milk thistle lower blood sugar and cholesterol levels in people with diabetes? Find out what a recent study showed in the Diabetes section of our Milk Thistle Supplements Review, which includes our Top Pick among milk thistle supplements.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
6/23/2023
Vitamin D & Diabetes Risk
In people with prediabetes, does taking vitamin D improve blood sugar regulation and decrease diabetes risk? Find out what a recent study showed in the Diabetes, insulin resistance and glucose control section of our Vitamin D Supplements Review.
Also see our Top Picks for vitamin D.
Clinical Update
10/07/2024
Curcumin for Diabetes?
Does taking curcumin improve blood sugar and insulin in people with type 2 diabetes? Find out what a recent study showed in the Insulin Resistance and Diabetes section of our Turmeric and Curcumin Supplements Review, which includes our Top Picks for curcumin supplements.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
4/14/2025
Vitamin D for Diabetes Prevention?
Did taking vitamin D for more than 5 years reduce the risk of type 2 diabetes among healthy older people in a major U.S. study? Find out in the Diabetes, insulin resistance and glucose control section of our Vitamin D Supplements Review.
Also see our Top Picks for vitamin D.
And see our article: Which supplements and foods can help lower or control blood sugar?
CL Answer
What is inositol hexaphosphate (IP6) and myo-inositol? Are they helpful in treating cancer or other conditions?
Inositol hexaphosphate (IP6): Find out if IP6 (also known as phytic acid) can prevent or treat cancer. Clinical evidence, dosage, safety and how IP6 differs from myo-inositol. Consumerlab.com's answer explains.

Product Review
Whole, Ground, Milled, and Cracker Flaxseed Review
High Levels of Cadmium Found in Flaxseed Products — Testing Expanded

Product Review
Iron Supplements Review (Iron Pills, Liquids and Chews)
See Which Iron Supplements Are CL's Top Picks for Different Needs

CL Answer
Does taking a laxative interfere with the absorption of vitamins or minerals?
Information on laxative interactions with other medications and supplements such as Maalox, antibiotics, and statins and diabetes medications.

Product Review
Noni Juice and Supplements Review Article
What Are the Benefits of Noni Juice? Get the Facts About Noni Juice and Supplements.

Recalls & Warnings
September 10, 2021
FDA Warns Ten Sellers of "Diabetes" Supplements
On September 7, 2021, the FDA issued warning letters to 10 supplement companies that made drug claims by promoting products to treat diabetes and/or lower blood sugar. Five of the products were sold on Amazon as well as on company websites. The products were promoted with statements such as
Product Review
Reishi Mushroom Supplements Review
Find the Best Reishi Mushroom Supplement. See How Reishi Supplements Differ.

CL Answer
Is canola oil good or bad for you? I heard it can be toxic.
Find out if canola oil is bad for you or good for you, and whether it helps heart health. Clinical evidence and how it compares to other oils.

Product Review
Vitamin E Supplements Review
Find the Best Vitamin E Supplement. Tests and Reviews of Popular Vitamin E Supplements & CL's Top Picks.
Product Review
Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, Boswellia, Collagen and Turmeric)
18% of Joint Health Supplements Failed to Pass Our Tests. See Which Passed or Failed, and Our Top Picks.

CL Answer
Dihydromyricetin (DHM) – Does It Help and Is it Safe?
Dihydromyricetin (DHM) is promoted for liver health, diabetes, lowering cholesterol, and as a hangover remedy. Find out if it works and whether it is safe.

CL Answer
Hemp Seeds: Health Benefits, Safety, and What To Consider When Picking a Product
Hemp seeds are promoted for heart health, diabetes, boosting testosterone, and other conditions, but do they work? Find out and learn about potential safety concerns.

CL Answer
What is TUDCA (tauroursodeoxycholic acid)? Does it have any proven health benefits, and is it safe?
TUDCA information, including its potential health benefits, safety, possible interactions, and cost.
CL Answer
Supplements to Take or Avoid When Using a GLP-1 Drug
Certain supplements may be helpful when taking a GLP-1 drug, but others should be used with caution or avoided. It may also be important to monitor levels of certain vitamins and minerals. Get the details.
CL Answer
What are trace minerals and do I need them?
Trace minerals include boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, zinc, and others. Find out what they do, how much of each trace mineral you should be getting, and who's most likely to be deficient in these minerals.
CL Answer
Can taking too much fish oil be dangerous?
Find out if too much fish oil can be dangerous, and if taking too much fish oil can cause effects such as suppressing the immune system, thinning the blood (increasing the risk of bleeding), increasing levels of LDL cholesterol and liver enzymes or increasing the frequency in episodes atrial fibrillation. ConsumerLab.com's answer explains.

CL Answer
What is graviola? Can it really help fight cancer?
Learn more about graviola, if it can really help fight cancer, and its safety.

CL Answer
What's the Best Way to Take Vitamin B-12? Dosage and Absorption Tips
Discover the optimal dosage and ways to take vitamin B-12, an essential nutrient, for better absorption and effectiveness and learn about possible side effects.
Product Review
Acetyl-L-Carnitine Supplements Review
Choose the Best Acetyl-L-Carnitine Supplement. Don't Overpay! CL Identifies Quality Acetyl-L-Carnitine Supplements at the Best Value.
.jpg?size=small)
Product Review
Green Coffee Bean Extract Supplements Review (for Weight Loss)
Choose the Best Green Coffee Bean Extract. 50% of Green Coffee Bean Extract Supplements Don't Deliver Expected Ingredients.

Product Review
Black Seed Oil Review
Find Out Which Black Seed Oil Supplements Passed or Failed Our Tests. See Our Top Picks.

Product Review
Prebiotic Supplements Review
You Can't Tell How Much Fiber Is Really In Most Prebiotics Without Testing

Product Review
Ginkgo (Ginkgo Biloba) Supplements Review
Choose the Best Ginkgo Biloba Supplement. Finding Real Ginkgo Isn't Easy — 60% Fail ConsumerLab's Tests of Quality.

Product Review
CLA (Conjugated Linoleic Acid) Supplements Review (for Slimming)
Choose the Best CLA Supplement. Not All CLA Supplements Contain What You Expect.

Product Review
Psyllium Fiber Supplements Review
Find the Best Psyllium Fiber Supplement and Avoid Lead.

Product Review
Rolled Oats and Steel-Cut Oats Review
Find the Best Rolled and Steel-Cut Oats -- Beware of Unexpected Gluten.

CL Answer
Can taking too much vitamin B-12 be dangerous? The label on my B-complex states it contains 50,000% of the Daily Value!
Find out the daily value for vitamin B-12 and the potential side effects of taking too much B-12. ConsumerLab explains why taking too much vitamin B-12 can be harmful.
CL Answer
Can white kidney bean extract lower blood sugar or help me lose weight?
Does white kidney bean extract help promote weight loss? Find out more including evidence from clinical studies and dosage.

CL Answer
Which vitamins and minerals should someone over 70 take?
Find out which vitamin and mineral supplements men and women age 70 and older should take, recommended intakes for vitamin D, B6, B12, iron, plus multis, vision supplements, protein and more.

CL Answer
Supplements for multiple sclerosis (MS): Do any supplements reduce symptoms or relapse?
Find out if vitamins or supplements such as ginkgo, fish oil, biotin, vitamins A, C, E, D, and B12, or selenium reduce symptoms or relapse in people with multiple sclerosis (MS).
CL Answer
Does osha root have health benefits and is it safe?
Osha root extracts are commonly promoted for congestion, coughs, throat irritation, and other uses. Find out if research supports these uses, and learn if osha root is safe.

Clinical Update
8/26/2012
Curcumin May Help Prevent Diabetes
Past research has suggested a potential role for curcumin (from turmeric) in blood sugar control. A recently published placebo-controlled study of curcumin given daily to people with prediabetes (those with blood sugar levels somewhat higher than normal) produced dramatic results in reducing the percentage of such individuals progressing to diabetes. Get the details in the latest update to ConsumerLab.com's Turmeric and Curcumin Supplements Review -- which includes quality ratings of products and other potential uses of curcumin. More >>
Clinical Update
4/15/2017
Probiotic Reduces Diabetes Risk In Pregnancy
Taking a certain type of probiotic during pregnancy reduced the risk of gestational diabetes, according to a new study. For details see the "What It Does" section of the Probiotic Supplements Review >>
Clinical Update
4/15/2017
Chocolate May Reduce Diabetes Risk
"Occasional" consumption of chocolate was associated with a lower risk of developing diabetes among certain postmenopausal women, according to new study. For details about this and other effects of chocolate, see the "What It Does" section of the Cocoa Powders, Dark Chocolate, Extracts, Nibs, & Supplements Review >>
Clinical Update
2/27/2018
Ginseng Role in Diabetes
Results of a recent study suggest that a form of ginseng can help control blood sugar in people with type 2 diabetes. Get the details in the What It Does section of the Ginseng Supplements Review.
Clinical Update
8/07/2018
Vitamin D and Blood Sugar Control
A recent study adds to the growing evidence that obtaining and maintaining adequate vitamin D levels can help with blood sugar control. For details about this and studies regarding vitamin D and diabetes, see the Diabetes, insulin resistance and glucose control section of the Vitamin D Supplements Review. (Also see our Top Picks for vitamin D supplements.)
Clinical Update
9/04/2018
Fish Oil & Diabetes
In a major study, fish oil was given to people with type 1 or type 2 diabetes for several years to see if it would reduce serious cardiovascular events, like heart attacks and stroke. Find out what happened in the Heart Attack and Stroke section of the Fish Oil Supplements Review. (Also see our Top Picks among fish oil supplements.)
Clinical Update
10/09/2018
Cocoa for Diabetes?
Cocoa flavanols may help control blood sugar, but do they offer benefits to people with diabetes? See what recent studies are finding in the What It Does section of the Cocoa and Dark Chocolate Review. (Also see our Top Picks among products).
Clinical Update
4/20/2016
Diabetes Cured With Low Cal Diet
A recent study found that type 2 diabetes was reversed in 40% of people who followed an 8-week, very low calorie diet consisting mainly of a diet shake. Get details about the study in the "What It Does" section of the Protein Powders and Drinks Review – for Body Building, Sports & Dieting >>
Clinical Update
7/12/2014
Vitamin D & Calcium for Diabetes Due to Pregnancy
A new study found supplementation with a combination of vitamin D and calcium improved fasting blood sugar levels, serum insulin levels, and insulin sensitivity in women with gestational (pregnancy-related) diabetes. For more details, including dosage, as well as our tests and comparisons of vitamin D and calcium supplements, see the update to the Vitamin D Supplements Review (Including Calcium, Vitamin K, Magnesium) >>
Clinical Update
8/21/2020
Caution With Melatonin & Diabetes
Research is raising concern regarding the use of melatonin by people with diabetes. For more details – as well as other concerns with melatonin – see the Concerns and Cautions section of the Melatonin Supplements Review. Also see our Top Picks for Melatonin.
Clinical Update
7/23/2019
Extra Virgin Olive Oil and Diabetes
Several studies have looked at the effects of consuming extra virgin olive oil on improving blood sugar control. The most recent study looked at whether it could delay the need for glucose-lowering medication in people with type 2 diabetes. Get the results in the What It Does section of the Extra Virgin Olive Oils Review. Also see our Top Picks among products.
Clinical Update
2/11/2020
Fish Oil for Diabetes?
There is mixed evidence as to whether fish oil helps people who have diabetes. Get the details, including those of a new study, in the What It Does section of the Fish Oil Supplements Review. Also see our Top Picks for fish oil.
Clinical Update
2/08/2020
Red Yeast Rice and Diabetes?
The use of statin drugs, like lovastatin (Mevacor) is associated with an increase in the risk of developing diabetes. But does red yeast rice carry the same risk? See what a large study found in the Concerns and Cautions section of the Red Yeast Rice Review. Also see our Top Pick for red yeast rice.
Clinical Update
4/23/2017
Olive Oil Lowers Diabetes Risk
Consuming a certain amount of olive oil daily reduces the risk of developing type 2 diabetes by 13%, according to a new study. Consuming more than this amount did not reduce the risk further. Get the details, and see our tests of popular oils, in the "What It Does" section of the Extra Virgin Olive Oils Review >>
Clinical Update
8/29/2019
Can Yogurt Reduce Diabetes Risk?
Increasing yogurt intake, particularly in place of certain other dairy foods, may decrease the risk of developing type 2 diabetes. For details about this and other effects of yogurt, see the Fermented Dairy Foods section of the Probiotics Supplements Review. Also see our Top Picks for probiotics.
Clinical Update
9/10/2019
Apple Cider Vinegar for Type 2 Diabetes?
Studies have shown that taking apple cider vinegar with or before a meal can reduce the subsequent rise in blood sugar. But does apple cider vinegar improve insulin sensitivity in people with type 2 diabetes? Find out what a recent study showed in the Blood Sugar Control section of the Apple Cider Vinegar Review. Also see our Top Picks for apple cider vinegar.
Clinical Update
8/19/2021
Diabetes and Fruit Intake
Eating whole fruits is associated with reduced risk of type 2 diabetes and better blood sugar control. But how much fruit is associated with greatest benefit, and what's the role of apples, bananas, and citrus fruits? See what a recent study showed. It's all in our article: Which supplements and foods can help lower or control my blood sugar?
Clinical Update
8/31/2021
Spirulina for Type 2 Diabetes?
Does taking spirulina help control blood sugar levels in people with type 2 diabetes? See what a study found in the What They Do section of our Greens and Whole Food Powders and Capsules Review.
Clinical Update
3/03/2021
Diabetes Risk With Selenium
High intake of selenium from the diet or supplements has been associated with increased risk of type 2 diabetes. Get the details in the Concerns and Cautions section of our Selenium Supplements Review.
Clinical Update
4/04/2021
Diabetes & Sardines
Can eating sardines reduce the risk of type 2 diabetes? See what a new study found in the What It Does section of our Tuna, Salmon & Sardines Review. Also see our Top Picks for sardines, canned salmon and tuna.
Learn about the effects of fish oil supplements on blood sugar and insulin sensitivity in our Fish Oil Supplements Review.
Clinical Update
3/30/2019
Apple Cider Vinegar & Diabetes
Can apple cider vinegar help control blood sugar in people with type 2 diabetes? See what a recent study found in the What It Does section of the Apple Cider Vinegar Review. Also see our Top Picks for apple cider vinegar.
Clinical Update
1/04/2022
Vitamin D for Diabetic Nerve Pain?
Can vitamin D supplementation reduce nerve pain or other symptoms of peripheral neuropathy, such as numbness and tingling, in people with type 2 diabetes? See what a new study found in the Diabetes section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
For information about other supplements that may help, see our answer to the question: Do any supplements, like Nervive, help with nerve pain, like sciatica or diabetic neuropathy?
Clinical Update
11/10/2023
Magnesium for Diabetes?
Does magnesium supplementation improve insulin sensitivity or blood sugar control in people with type 2 diabetes? See what a recent study found in the our Magnesium Supplements Review, which includes our Top Picks for magnesium.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
4/22/2022
Cinnamon for Lowering Blood Sugar?
Does taking cinnamon powder or extract help control blood sugar levels in people with prediabetes or type 2 diabetes? See what the latest study, and earlier studies, have found in the Blood sugar, type 2 diabetes and prediabetes of our Cinnamon Supplements Review.
Also see: Which supplements and foods can help lower or control my blood sugar?
Clinical Update
3/25/2022
Multivitamin for Diabetes
We learned this week that our Top Pick multivitamin for people with diabetes has been discontinued. See our Update about this and alternatives. Also see our other Top Picks among multivitamins.
Clinical Update
3/29/2022
Mulberry Leaf for Diabetes?
Can taking mulberry leaf extract reduce blood sugar or improve insulin resistance in people with type 2 diabetes? Find out what a recent study suggests in our updated answer to the question: Which supplements and foods can help lower or control my blood sugar?
Clinical Update
8/11/2023
Kombucha for Diabetes?
Does drinking kombucha help lower blood sugar levels in people with type 2 diabetes? Find out what a recent study showed in the Kombucha section of our Probiotic Supplements Review.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
2/07/2023
Whey Protein for Diabetes
Whey protein seems to help control blood sugar levels, and a recent study suggested that it may do more than just slow gastric emptying and increase insulin levels. Get the details in the Diabetes section of our Protein Powders and Shakes Review. Also see our Top Picks for whey protein.
Clinical Update
8/08/2025
Turmeric for Heart Health?
Does turmeric lower heart risks in people with diabetes? See what a recent study found in the Insulin Resistance and Diabetes section of our Turmeric and Curcumin Supplements Review, which includes our Top Picks for turmeric and curcumin.
Clinical Update
10/28/2024
Wellmune for Diabetes?
Does taking Wellmune (which contains beta-glucan) improve blood sugar control in people with type 2 diabetes? Find out what a recent study showed in our article about supplements and foods for controlling blood sugar.
Clinical Update
8/29/2024
Sucralose & Diabetes
Does using sucralose (Splenda) instead of sugar in coffee and tea improve blood sugar control in people with type 2 diabetes? Find out what a recent study showed in our article about sugar substitutes, which includes our Top Picks among sweeteners.
CL Answer
Which supplements help with erectile dysfunction?
Supplements for erectile dysfunction. Find out which supplements can help with ED, including ginseng, L-arginine, and maca.

CL Answer
Which supplements reduce the risk of stroke? Which increase the risk of stroke?
Find out which supplements (may help reduce the risk of stroke/might increase the risk of stroke.)

CL Answer
Are there drug interactions with magnesium supplements?
Find out about interactions between magnesium supplements and over-the-counter antacids and laxatives such as Maalox, antibiotics, and statins and diabetes medications. ConsumerLab’s answer explains.

CL Answer
Which supplements can help lower my triglycerides?
Find out whether fish oil supplements, or plant-based oil supplements, such as flaxseed oil and echium oil are best for lowering triglyceride levels. ConsumerLab.com's answer explains.
Product Review
Rhodiola Rosea Supplements Review
Do Rhodiola Supplements Help With Depression and Anxiety? Find Out and See Which Rhodiola Supplements Provide the Best Quality & Value.

Product Review
Melatonin Supplements Review
Trouble Sleeping? See CL's Tests of Melatonin Supplements and Top Picks.
CL Answer
Immuno 150: Does It Boost Immunity?
Immuno 150 by Exceptional Health Products is promoted for boosting the immune system, but does it work and is it worth its price? Find out.

Product Review
Vitamin K Supplements Review (Including Calcium, Vitamin D, Magnesium & Boron)
Find Out If It's Worth Taking Vitamin K and Which Products Are Best
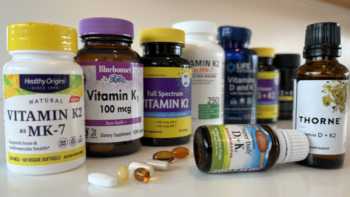
Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

Product Review
L-Arginine Supplements Review
Choose the Best L-Arginine Supplement. Find Out Which L-Arginine Supplement Passed CL's Tests.

Product Review
Cranberry Juices and Supplements Review
CL's Tests Show Which Cranberry Juices and Supplements Are Best and Cost the Least

Product Review
Aloe Juices, Gels, and Supplements Review
How Much Aloe is Really in Aloe Products? Find Out and See Our Top Picks.

CL Answer
Are cranberry supplements helpful for men? Can they help with symptoms of an enlarged prostate, prostatitis, or urinary tract infections?
Learn more about how cranberry pills, cranberries, cranberry juice and supplements can help prevent urinary tract infections.

CL Answer
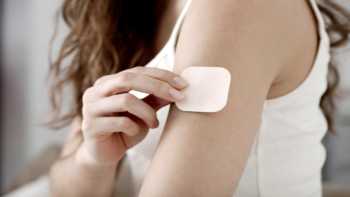
Do vitamin patches, such as for B12 or multivitamins, really work? How about those from PatchMD?
Do vitamin B12 Patches like PatchMD really work? Can you absorb vitamin B12 through patches?
CL Answer
Does Provitalize work for weight loss and is it safe?
Find out if taking Provitalize, a probiotic supplement that also contains turmeric extract, moringa leaf extract, curry leaf extract and piperine (BioPerine), relieves joint pain, decreases abdominal fat, or helps with weight loss.

CL Answer
Do detox supplements work and are they safe?
Detox supplements are promoted for removing toxins such as heavy metals from the body, but does research support their use and are such supplements safe?

CL Answer
Risks of Too Many Vitamins & Supplements
Taking too many vitamin supplements -- or taking too much of a particular supplement -- can have short and long-term adverse effects.

CL Answer
Is it safe to take arginine, or other supplements for sexual enhancement, while taking Viagra or Cialis?
When taking Cialis or Viagra, is it safe to take arginine or other sexual enhancement supplements?

CL Answer
Is P-5-P really better than "regular" vitamin B6?
Learn more about pyridoxal-5-phosphate (P-5-P) and vitamin B6.

CL Answer
Which supplements or foods can help lower cholesterol and keep my heart healthy? Are there any to avoid?
Information about heart health supplements & vitamins that help with cholesterol, including sterol esters, CoQ10, and vitamin D, and which may be bad for the heart, like calcium.

Product Review
Pomegranate Juice and Supplements Review Article
What Are the Benefits of Pomegranate Juice and Supplements? Find Out What Pomegranate Can and Cannot Do For Your Health.

Product Review
Vitamin C Supplements Review
Find the Best Vitamin C Supplements

News Release
February 15, 2024
Unveiling the Truth About Green Tea’s EGCG Content
White Plains, NY, February 15, 2024 — ConsumerLab.com, a trusted name in health and wellness, sheds light on the EGCG (epigallocatechin gallate) content in green tea.
CL Answer
What are the health benefits of palmitoylethanolamide (PEA), and is it safe?
Palmitoylethanolamide (PEA) is commonly promoted for its anti-inflammatory and pain-relieving effects. Find out if it works.

CL Answer
Dandelion Tea & Supplements: Health Benefits and Safety
Find out about the potential health benefits of dandelion tea and supplements, as well as safety, side effects, and potential drug interactions.

Product Review
Muscle & Workout Supplements Review (Creatine and Branched-chain Amino Acids)
Do Creatine and BCAAs Really Improve Strength and Recovery?

CL Answer
Do "lite" salts and salt substitutes containing potassium help lower blood pressure and reduce heart risks? Are they safe?
Find out if replacing regular salt with "lite" salt or salt substitutes, such as Morton Lite Salt, Morton Salt Substitute, NoSalt Original Sodium Free Salt Alternative, and MySalt, helps to reduce blood pressure, or decrease the risk of heart attack and stroke. Plus, learn about the safety and side effects associated with salt substitutes, drug interactions, and more.

Recalls & Warnings
July 25, 2013
Beware of Dietary Supplements With Claims To Treat Diabetes, FDA Warns
On July 23, 2013, the FDA issued a warning to consumers not to buy or use dietary supplements that are promoted to treat diabetes.
CL Answer
Are Balance of Nature products good dietary supplements?
Do Balance of Nature supplements "Fruits" and "Veggies" supplements really provide the daily recommended servings of fruits and vegetables, and are they worth the cost? Find out.

CL Answer
8 Red Flags to Watch Out For When Buying Vitamins & Supplements
When it comes to buying vitamins and supplements, it's buyer beware. ConsumerLab explains eight red flags to look out for on labels when choosing and buying vitamins or supplements, including claims of "FDA Approved" laboratories, what to look for in the ingredient list, and more.

CL Answer
What is C15:0 fatty acid (found in fatty15), and does it have health benefits?
C15:0 fatty acid in fatty15 is promoted for “healthier hair & skin, balanced metabolism, and deeper sleep,” as well as “slowing the aging process.” Find out if it works.

CL Answer
What are the possible health benefits of fenugreek, and is it safe?
Fenugreek is promoted for boosting testosterone, increasing breastmilk production, lowering blood sugar, improving symptoms of polycystic ovary syndrome (PCOS), and other conditions, but does it work? Find out.

CL Answer
Which supplements may help with PCOS (polycystic ovarian syndrome)?
Many supplements are promoted to help treat symptoms of PCOS (polycystic ovarian syndrome), but do they really work? See the clinical evidence for inositol (myo-inositol and D-chiro-inositol), alpha lipoic acid, vitamin B-12, folic acid, vitamin D and more.

CL Answer
What is Perque Bone Guard Forté? It is safe and effective for bone health?
Find out about the ingredients in Perque Bone Guard Forté, including which ingredients may be beneficial for osteoporosis and bone health and which may be a safety concern.

CL Answer
Desiccated Beef Liver and Beef Organ Supplements: Nutrition & Safety
Description of desiccated beef liver, including what it is used for, its nutrient content, and safety concerns.

CL Answer
Do any supplements help for varicose veins or chronic venous insufficiency?
Find out which supplements help for varicose veins and chronic venous insufficiency, including citrus bioflavonoids and grape seed extract.

CL Answer
8 Supplements That May Help Treat Peptic Ulcers – And Some to Avoid
Peptic ulcers caused by H. pylori infection are typically treated with antibiotics, but certain supplements may also help. Find out which 8 supplements might be beneficial - and which should be avoided.

CL Answer
Do Any Supplements Improve Dry Mouth (Xerostomia) or Make It Worse?
Find out if any supplements, topical formulations (patches, rinses, lozenges, toothpastes, sprays, gels or gums), or lifestyle modifications reduce dry mouths symptoms.

News Release
September 14, 2023
Best and Worst Berberine and Goldenseal Supplements, According to ConsumerLab Tests
White Plains, New York, September 14, 2023 — Recent ConsumerLab tests revealed problems with nearly 45% of the berberine and goldenseal supplements selected for testing. Powders and capsules containing goldenseal (which naturally contains berberine) were particularly likely to have issues.
News Release
July 10, 2019
Best Selenium Supplements Identified by ConsumerLab
White Plains, New York, July 10, 2019 — Selenium is an essential mineral important for proper immune and thyroid function but taking a selenium supplement is often not necessary and, in some people, may increase the risk of cancer or diabetes.
CL Answer
Do Probiotics, Such as Those by Bioma and Pendulum, Boost GLP-1 to Help with Weight Loss?
Discover the differences between probiotics and GLP-1 agonists for blood sugar and weight management. Products discussed include Pendulum Glucose Control, Pendulum GLP-1 Probiotic, and Bioma Gut Health Probiotics.
Recalls & Warnings
October 29, 2019
Publisher Sued for Promoting "Phony Diabetes Cure"
On October 24, 2019 the FTC filed a lawsuit against the publisher of books, newsletters, and other publications aimed at seniors that falsely promise to cure type 2 diabetes.
News Release
January 03, 2019
ConsumerLab Tests Reveal the Best Protein Powders, Shakes and Drinks -- 20% of Protein Powders and Drinks Fail CL's Tests of Quality
White Plains, New York, January 3, 2019 — Combined with resistance exercise, protein supplements can help athletes build muscle and older people prevent muscle loss. They can also help people with diabetes maintain blood sugar levels when consumed as part of a low-calorie diet.
News Release
July 10, 2018
Best Chromium Supplements Identified by ConsumerLab
White Plains, New York, July 10, 2018 — Chromium supplements are promoted to help control blood sugar in people with type 2 diabetes and with weight loss.
News Release
December 12, 2017
Berberine and Goldenseal: Avoid Fakes! -- ConsumerLab.com Testing Reveals the Best and Worst Supplements
White Plains, New York, December 12, 2017 — Berberine, a compound found in goldenseal root, may help to lower blood sugar in certain people, and may have other potential benefits. However, ConsumerLab.
News Release
June 21, 2017
Which Magnesium Is Best? ConsumerLab.com Tests Popular Brands and Reveals Its Top Pick
White Plains, New York, June 21, 2017 — Magnesium supplements are among the most popular supplements, but do you need to take one and, if so, which is best? To find out, ConsumerLab.com reviewed the clinical evidence, and purchased and tested popular magnesium supplements sold in the U.S.
News Release
April 04, 2017
ConsumerLab.com Reveals Best and Worst Turmeric and Curcumin Supplements & Spices -- 31% Fail ConsumerLab.com Tests of Quality
White Plains, New York, April 4, 2017 — Turmeric and its key compound, curcumin, may be help reduce symptoms of inflammation in rheumatoid arthritis and osteoarthritis and may be helpful in treating depression, diabetes, and indigestion.
News Release
December 06, 2016
Which Brands of Alpha Lipoic Acid are Best? -- ConsumerLab.com Tests Lipoic Acid Supplements for Quality, Identifying Top Picks
White Plains, New York, December 6, 2016 — Alpha-lipoic acid supplements may improve insulin sensitivity and blood sugar control in people with type 2 diabetes, reduce symptoms of diabetic peripheral neuropathy, and enhance weight loss when dieting.
News Release
October 11, 2016
What Are the Benefits of Resveratrol and Which Brands Are Best? -- ConsumerLab.com Reviews the Evidence and Tests the Quality of Popular Resveratrol Supplements
White Plains, New York, October 11, 2016 — Resveratrol supplements have been popular since 2006, when studies in animals showed "life-extending" and "endurance-enhancing" effects, among other potential benefits.
News Release
July 05, 2016
80% of Milk Thistle Supplements Fail ConsumerLab.com Tests of Quality -- Most Found to Be Low in Key Component
White Plains, New York, July 5, 2016 — Milk thistle supplements naturally contain silymarin compounds which may be helpful in certain liver diseases as well as type 2 diabetes. However, recent ConsumerLab.
News Release
January 12, 2016
Problems Found with 32% of Multivitamin/Multimineral Supplements -- ConsumerLab.com Identifies Good Choices for Men, Women, and Children
White Plains, New York, January 12, 2016 — You can't always judge a supplement by its label -- or by its price, according to a new report from ConsumerLab.com, which recently tested dozens of multivitamin/multimineral supplements.
News Release
January 05, 2016
Cinnamon Spices and Supplements Found to Vary Widely in Levels of Potentially Helpful and Harmful Compounds Reports ConsumerLab.com
White Plains, New York, January 5, 2016 — Cinnamon supplements may be helpful for controlling blood sugar in people with type 2 diabetes or prediabetes, possibly due to compounds known as proanthocyanidins or PACs.
CL Answer
Energy Gels: What Are They Used For and Do They Contain Their Labeled Ingredients?
Energy gels are a convenient on-the-go carbohydrate source, but some products may not contain the amount of carbohydrates stated on the label.

CL Answer
Do any supplements help with fatty liver disease? Are some diets more beneficial than others?
Find out if supplements such as vitamin E, fish oil, milk thistle, curcumin, choline, probiotics, vitamin D, reishi mushroom, or extra virgin olive oil are beneficial for fatty liver disease and learn which diet (Mediterranean, ketogenic, DASH, or fasting diets) seems to have greatest benefit.
CL Answer
Which supplements can help to lower blood pressure?
Supplements to lower blood pressure, including CoQ10, vitamin C, fish oil, and olive oil, are all explored to find out what works.

CL Answer
My doctor told me to stop taking supplements because my kidney function was low. After stopping, my kidney function returned to normal. Can taking a lot of supplements really damage the kidneys?
Find out which supplements may damage your kidneys. ConsumerLab explores possible kidney side effects of supplements including chromium, creatine, and vitamin C.

CL Answer
What B-vitamin complex do you recommend for older people?
Vitamin B complex supplement recommendations, benefits and top rated brands for older people and all ages.

CL Answer
My HDL (good) cholesterol level is low. Should I take niacin to raise my HDLs?
Learn more about niacin and find out if it can raise HDL ("good") cholesterol levels.

Recalls & Warnings
December 26, 2024
Blood Sugar Supplement Recalled Due to Undeclared Metformin and Glyburide
On December 16, 2024, Shoppers- Plaza recalled all lots of Fouzee Sugarlin Herbal Formula capsules because they were found to contain undeclared metformin and glyburide.
Clinical Update
11/14/2024
Hemp Seed Benefits?
What are hemp seeds and does eating them help with heart health, diabetes, or boosting testosterone? Find out in our article about the benefits, safety, and what to consider when picking a product.
Also see our test results for:
Clinical Update
2/24/2025
Goldenseal, Metformin Interaction
Be aware goldenseal might reduce the absorption of the diabetes medication metformin, according to a recent study. For details, see the Concerns and Cautions section of our Berberine and Goldenseal Supplements Review, which includes our Top Picks for berberine and goldenseal.
Clinical Update
3/03/2025
Herb for Hair?
Does taking jiaogulan (Gynostemma pentaphyllum) improve hair thickness? Find out what a recent study showed in our article about supplements for hair.
Clinical Update
9/25/2025
Fish for Retinopathy?
Omega-3 rich foods like fish may lower diabetic retinopathy risk, but benefits may be greater for people with type 2 than type 1 diabetes, according to a recent analysis. Get the details in the Retinal Disease section of our Fish Oil Supplements Review, which includes our Top Picks among fish oil supplements.
Clinical Update
3/07/2023
Amla Extract for Blood Sugar?
Did taking amla extract reduce fasting blood sugar or HbA1c in people with type 2 diabetes? Find out in our article about the health benefits of amla extract.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
12/16/2022
Gymnema & Sugar Cravings
Did supplementing with gymnema (which may alter taste perception) curb sugar cravings and reduce food intake in a recent study? Find out in our updated CL Answer about supplements for reducing appetite and weight.
Also see our CL Answer about supplements for blood sugar control to learn about use of gymnema in people with diabetes.
Clinical Update
1/31/2023
B12 Deficiency & Metformin
If you take metformin to treat diabetes, be aware that you are at higher risk of vitamin B12 deficiency. Recent laboratory research suggests a possible reason for this, as discussed in the ConsumerTips section of our B Vitamins Review. Also see our Top Picks among B12 supplements.
Clinical Update
8/02/2022
Metformin and B-12 Deficiency
If you take metformin for diabetes, be aware that you are at risk for vitamin B-12 deficiency. See the latest guidelines to monitor for this in the B-12 section of our B Vitamin Supplements Review.
Clinical Update
5/02/2023
Cinnamon for Lowering Blood Sugar?
Did taking capsules of true (Ceylon) cinnamon reduce fasting blood sugar or HbA1c in people with type 2 diabetes? See what a new study found in the What It Does section of our Cinnamon Supplements Review, which includes our Top Picks among cinnamon supplements and spices.
Clinical Update
6/06/2023
Berberine's New Popularity
Berberine is being called "nature’s Ozempic" – referring to the type 2 diabetes drug also used for weight management. But is there evidence to support this? See our Berberine Review to find out what it can do, if it's safe, how products rate on quality, and our top choices.
Clinical Update
6/09/2023
Menthol Rubs for Pain?
Do menthol rubs like Biofreeze and Vicks VapoRub really reduce pain, including diabetes-related nerve pain, and are rubs with capsaicin any better?
Clinical Update
8/22/2023
Green Tea and Blood Sugar
A laboratory study showed how green tea might help control blood sugar. Get the details in the Blood Sugar Control/Diabetes section of our Green Tea Review, which includes our Top Picks for green tea.
Also see: Which supplements and foods can help lower or control blood sugar?
Clinical Update
8/22/2023
Melatonin and Blood Sugar Control
Melatonin supplements may affect blood sugar control and insulin function in people with type 2 diabetes, according to recent studies. Get the details in the Concerns and Cautions section of our Melatonin Supplements Review, which includes our Top Picks for melatonin.
Clinical Update
6/27/2023
Allulose & Blood Sugar
Does using the sweetener allulose along with sugar help lower blood sugar and insulin levels after a meal? Find out what a recent study showed in the Diabetes section of our article about sweeteners.
Also, find out what the main ingredient is in BetterStevia Organic Glycerite. Hint: It’s not stevia.
Clinical Update
3/12/2024
Green Tea & Blood Sugar
Does green tea reduce fasting blood sugar levels? See what a recent study showed in the Blood Sugar Control/Diabetes section of our Green Tea Review, which includes our Top Picks for green tea.
Clinical Update
12/12/2023
Fish Oil & Retinopathy
Does taking fish oil decrease the risk of developing retinopathy in people with diabetes? See what a major study found in the Retinal Disease section of our Fish, Krill, and Algal Oil Supplements Review. Also see our Top Picks among omega-3 supplements.
Clinical Update
4/19/2024
Acetyl-L-Carnitine for Nerve Pain?
Is acetyl-L-carnitine recommended to reduce nerve pain in people with diabetes? Find out in the What It Does section of our Acetyl-L-Carnitine Supplements Review.
Also see: Do any supplements, like Nervive, help with nerve pain, like sciatica, diabetic neuropathy or postherpetic neuralgia?
Clinical Update
6/30/2013
Vitamin D & Depression
Women with depression and type 2 diabetes were given high-dose vitamin D for several weeks in a small, pilot study. By the end of the study, vitamin D levels in the blood had risen considerably and mood was significantly improved, as was blood pressure. Although just a pilot study, this is not the first study to suggest that vitamin D may help with depression. For more details, as well as test results and comparisons for 55 vitamin D supplements, see the updated Vitamin D Supplements Review >>
Clinical Update
3/11/2012
When Not to Take Selenium
A researcher has advised not to take selenium if your selenium level is already adequate. Supplementation in people with adequate to high serum levels of selenium may increase the likelihood of developing type 2 diabetes. For details, see the update to the Concerns and Cautions section of the Cancer Supplements Review. More >>
Clinical Update
6/24/2012
Fish Oil for Strokes and Heart Attacks?
A recent study with fish oil supplements found no reduction in strokes and heart attacks among people with diabetes and poor glucose control. Does this mean fish oil doesn't help with cardiovascular disease? Not necessarily. See the updated Fish Oil (Omega-3) Supplements Review. More >>
Clinical Update
8/19/2012
Low Vitamin D Levels and Obesity -- A Dangerous Combination
A new study links low blood levels of vitamin D in obese individuals to a greatly increased risk of insulin resistance (a condition linked to type 2 diabetes). The results add to the growing list of reasons to make sure you maintain a sufficient level of vitamin D. Get the details in the latest update to ConsumerLab.com's Vitamin D Supplements Review. More >>
Clinical Update
8/19/2012
Can Resveratrol Help Control Blood Sugar?
A new clinical study suggests potential benefits for people with type 2 diabetes taking one of the resveratrol products evaluated by ConsumerLab.com. Get the details in the latest update to ConsumerLab.com's Resveratrol Supplements Review. More >>
Clinical Update
1/11/2011
Insulin Sensitivity and Magnesium
A recent study found that magnesium supplementation improved fasting glucose levels in insulin insensitive, obese people. While a similar effect has been seen in magnesium deficient people, the new study found benefit in people who had normal magnesium levels. The research suggests that magnesium may help prevent the onset of type 2 diabetes in some people. See the Magnesium Supplements Review for the type and dose of magnesium used and more >>
Clinical Update
4/13/2014
Resveratrol for Glucose Control
A recent review of clinical studies shows that resveratrol can help with glucose control in people with type 2 diabetes. However, it is not the general fountain of youth originally hoped. For more information about resveratrol, including our tests of resveratrol supplements, see the updated Resveratrol Supplements Review >>
Clinical Update
11/14/2014
Ginseng for Lower Blood Sugar
A new study found that a certain kind of ginseng helps lower blood sugar levels in people with impaired fasting glucose. For more about this study and related studies on blood sugar and diabetes with other types of ginseng (which have had mixed results), see the Ginseng Supplements Review >>
Clinical Update
11/22/2014
Turmeric for Memory
A new study shows a modest benefit of turmeric on short-term memory among older individuals with pre-diabetes. Details about the study and dosage used, as well as results of our tests of popular products, are found in the Turmeric and Curcumin Supplements Review >>
Clinical Update
3/07/2015
Vitamin D Helps in Pregnancy
A new study showed that giving vitamin D to women with low levels of vitamin D resulted in a dramatic reduction in pre-term labor, pre-eclampsia, and gestational diabetes compared to untreated women. For details, see the Vitamin D Supplements Review >>
Clinical Update
6/24/2015
Problem with Too Much Krill Oil
Researchers recently gave overweight men a very large daily amount of krill oil (with a smaller amount of fish oil) with the hope it would improve their insulin sensitivity. Unfortunately, the opposite happened, suggesting that the oil could increase the risk of diabetes and cardiovascular disease. Details about the study, including dosage, are found in the "Concerns and Cautions" section of the Fish Oil and Omega-3 Fatty Acid Supplements Review (Including Krill, Algae, Calamari, Green-lipped Mussel Oil) >>
Clinical Update
3/15/2017
Anti-Inflammatory Effect of Juice Plus+?
Chronic systemic inflammation is a risk factor for cardiovascular disease, diabetes, and cancer. A recent clinical trial assessed the product Juice Plus+ for its ability to reduce this inflammation in obese men and women. The abstract of the published study indicates that it works, but the results tell a different story. For details, see the "What It Does" section of the Greens and Whole Foods Powders, Pills, and Drinks Review (which includes our tests of products) >>
Clinical Update
3/29/2017
Vitamin E/Selenium & Alzheimer's
A long-term study among men in the U.S. found that giving high doses of vitamin E and/or selenium did not significantly affect their risk of developing dementia. The study was part of a larger study which found that these supplements increased the risk of prostate cancer, type 2 diabetes, hair loss, and dermatitis. For details, see the "Alzheimer's disease/dementia" section of the Selenium Supplements Review >>
Clinical Update
8/30/2016
Resveratrol for Blood Sugar?
Despite preliminary studies which found resveratrol to help lower blood sugar in people with type 2 diabetes, a larger long-term study found no benefit. Find out more in the "What It Does" section of the Resveratrol Supplements Review >>
Clinical Update
7/12/2014
Whey Protein for Blood Sugar Control?
Drinking a whey protein shake before a meal improves blood sugar and insulin response in men and women with type 2 diabetes, according to a new, preliminary study. Get more details, plus our tests of protein supplements, in the updated Protein Powders and Drinks Review >>
Clinical Update
2/12/2019
Vitamin C for Blood Sugar Control
High-dose vitamin C was found to help control blood sugar levels in people with type 2 diabetes in a recent study. For details, see the What It Does section of the Vitamin C Supplements Review. Also see our latest Top Picks for vitamin C.
Clinical Update
1/31/2021
B12 for Diabetic Neuropathy?
Can high-dose vitamin B12 improve nerve function and reduce pain in people with neuropathy due to type 2 diabetes? See what a new study found in the B12 section of the B Vitamin Supplements Review. Also see our Top Picks for vitamin B12.
Clinical Update
5/04/2021
Glutathione for Blood Sugar?
Does taking glutathione improve blood sugar control in people with type 2 diabetes? Find out what a recent study showed in our answer to the question: Do glutathione supplements work to prevent aging or for other conditions?
Clinical Update
7/31/2021
Gynostemma for Weight?
Can taking Gynostemma pentaphyllum extract help reduce weight? We've added information about this to our article about Gynostemma (which is also promoted for diabetes and high cholesterol treatment).
Clinical Update
10/03/2020
Vitamin C for Foot Ulcers?
Can taking vitamin C help heal foot ulcers in people with diabetes or vascular disease? Find out what a new study showed in the What It Does section of our Vitamin C Supplements Review. Also see our Top Picks for vitamin C.
Clinical Update
10/24/2020
Vitamin D & Pregnancy
Maintaining sufficient blood levels of vitamin D during pregnancy is associated with better health for both mother and baby, including a decreased risk of gestational diabetes, according to a new study. Get the details in the Pregnancy section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
2/04/2017
Many Benefits of Magnesium
An analysis of studies involving more than one million people found that rates of stroke, heart failure, type 2 diabetes, and death were lower when intakes of magnesium were higher. For details see the "What It Does" section of the Magnesium Supplements Review (which includes our tests of products) >>
Clinical Update
1/04/2020
Curcumin for Blood Sugar Control?
Preliminary research suggests that curcumin (from turmeric) taken with meals can reduce the increases in blood sugar and insulin levels that occur after eating. But does curcumin help people with pre-existing type 2 diabetes? See what a recent study found in the What It Does section of the Turmeric and Curcumin Supplements & Spices Review. Also see our Top Picks among turmeric and curcumin products.
Clinical Update
10/20/2016
Vitamin D Sweet Spot
Having the right blood levels of vitamin D has many proven benefits, but too little and too much vitamin D can be problematic. A new study shows this also to be true in people with diabetes with regard to a type of nerve damage that can affect blood pressure and heart rate. For details see the "What It Does" section of the Vitamin D Supplements Review >>
Clinical Update
6/26/2018
Ubiquinol for Blood Sugar Control?
Does giving ubiquinol (the active form of CoQ10) to people with type 2 diabetes help with blood sugar control? Find out in the What It Does section of the CoQ10 and Ubiquinol Supplements Review. (Also see our Top Picks among CoQ10 and ubiquinol supplements.)
Clinical Update
2/08/2020
Glucosamine and Blood Sugar
Glucosamine (used to treat osteoarthritis) is a type of sugar, so does its daily use affect blood sugar levels and one's risk of developing diabetes? Find out in the Concerns and Cautions section of the Joint Health Review. Also see our Top Picks for Glucosamine, Chondroitin, MSM, and Boswellia supplements.
Clinical Update
7/21/2020
Cinnamon for Prediabetes?
Does taking a daily cinnamon supplement help control blood sugar in people with pre-diabetes? See the results of a recent study in the What It Does section of the Cinnamon Supplements & Spices Review. Also see our Top Picks among cinnamon supplements and among cinnamon spices.
Clinical Update
11/16/2018
Probiotic Lowers Eczema Risk in Children
The risk of eczema in children was reduced by 54% when a specific probiotic was taken by mothers during pregnancy and then by their infants, according to a new study. The probiotic also reduced the incidence of gestational diabetes among the mothers. For details, see the What It Does section of the Probiotic Supplements Review. (Also see our Top Picks for probiotics.)
Clinical Update
10/15/2019
Vitamin D & Blood Sugar Control
Maintaining moderate levels of vitamin D may aid blood sugar control, but, according to a recently published study, high-dose vitamin D supplementation may not help. For details, see the Diabetes, insulin resistance and glucose control section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
10/23/2021
Vitamin K and Coronary Artery Calcification
Can supplementing with vitamin K1 slow coronary artery calcification? See what a new study among people with diabetes found in the What It Does section of our Vitamin K Supplements Review. Also see our Top Picks among vitamin K supplements.
Recalls & Warnings
January 04, 2024
Lone Star Botanicals Warned by FDA for Manufacturing Violations
On November 26, 2023, the FDA issued a Warning Letter to Lone Star Botanicals, Inc.
CL Answer
Is ingesting colloidal silver helpful for any condition and is it safe to use?
Find out if there is evidence showing colloidal silver can help to treat infections, boost the immune system, help with arthritis and more, plus find out if colloidal silver can cause blue skin or other side effects.

CL Answer
When taking a statin drug like Lipitor or Crestor, are there supplements I should avoid or take?
Learn about the interactions between certain supplements and atorvastatin (Lipitor), rosuvastatin (Crestor), and other cholesterol-lowering statins.
CL Answer
What is Pycnogenol, does it work, and is it the same as other pine bark extracts?
Learn more about Pycnogenol, including evidence from clinical studies on blood clots, osteoarthritis, cognition, vision, tinnitus, and the skin and safety.

CL Answer
Can beetroot juice or supplements lower blood pressure or improve exercise performance, and are they safe?
Learn more about beetroot juice and beetroot supplements and if beetroot can really help lower blood pressure.

CL Answer
Do any supplements help with restless legs syndrome?
Find out whether supplements or vitamins like iron, magnesium, vitamin E and others can help for restless leg syndrome (RLS). ConsumerLab.com's answer explains.

CL Answer
Is it possible to take too much vitamin C?
Find out the daily value of vitamin C, along with possible side effects of excessive vitamin C intake. ConsumerLab explores the topic of proper vitamin C supplement usage, and why taking too much can be harmful.
CL Answer
Is topical menthol, such as Biofreeze, safe and effective for pain relief?
Menthol is commonly used in topical creams and gels for pain relief. Find out if it works and if it's safe.
Recalls & Warnings
November 06, 2023
FDA Warns Consumers Not to Use Dr. Ergin’s SugarMD Advanced Glucose Support, Found to Contain Prescription Drugs
On November 3, 2023, the FDA warned consumers not to purchase or use Dr. Ergin’s SugarMD Advanced Glucose Support products after FDA laboratory analysis found the supplement to contain glyburide and metformin.
CL Answer
What are the health effects of apigenin and is it safe?
Apigenin, a flavonoid compound, is promoted for sleep, depression, cancer, and other conditions. Find out if it works and if it's safe.

CL Answer
Does red and near infrared light therapy reduce pain, improve skin or cognition, or have other benefits? Is it safe?
Red light and near infrared light therapy devices are promoted for numerous conditions, including acne, fibromyalgia, pain, cognitive function and other uses. Find out if these devices work and if they are safe.

CL Answer
Which supplements can help treat anemia? Do any supplements cause a low red blood cell count?
Find out which vitamin and nutrient supplements can help prevent and treat anemia in people who are nutrient deficient and learn which supplements may cause low blood cell counts, especially when used in excess.

CL Answer
Can fisetin, also called Cognisetin and Novusetin, really improve memory?
Fisetin (Cognisetin and Novusetin) information, including evidence from clinical studies on memory improvement, brain health, dosage, and pricing.

CL Answer
Is sublingual vitamin B-12, which is placed under the tongue and allowed to dissolve, really better than the pill form?
Learn the difference between sublingual vitamin B-12, vitamin B-12 pills and more through information including clinical evidence.

News Release
October 14, 2015
Is Matcha a Better Form of Green Tea? ConsumerLab.com Answers the Question
White Plains, New York, October 14, 2015 — For the past three years, ConsumerLab.com has been testing green tea products, including tea bags, bottled drinks, dietary supplements, and even a K-Cup®.
News Release
May 21, 2013
Green Teas Vary in Strength and Amount of Lead Contamination, According to ConsumerLab.com
White Plains, New York, May 21, 2013 — If you drink green tea for your health, be aware that the catechin and caffeine levels can vary by more than 240% across products. Some also contain significant amounts of lead in their tea leaves. This is according to recent tests by ConsumerLab.
Recalls & Warnings
October 11, 2023
Family Sentenced to Over 12 Years in Prison for Selling Dangerous “Bleach” Miracle Mineral Solution as “COVID Cure”
Four Florida men who distributed the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions have been sentenced to 5 to 12 years in prison for conspiring to defraud the United States.
CL Answer
Can taking too much vitamin B-6 be dangerous?
Find out if multivitamins contain excessive amounts of B-6, how much B-6 is too much, and symptoms of toxicity and overdose.

Recalls & Warnings
August 16, 2023
FDA Warns Hekma Center, LLC for Promoting Products to Treat Anemia, Diabetes, Depression, & More
On June 2, 2023, the FDA issued a warning letter to Hekma Center, LLC following review of the company’s website and social media, which found statements about the company’s Natural Supplements for Anemia, Lymf (Galium aparine), Natural Supplements for Cardiomyopathy, Magic1 (Moringa ...
Clinical Update
4/01/2012
Vitamin D and the Risk of Death
In a six-year study of over 10,000 people in Kansas, the risk of death was found to be 164% higher among those with lower levels of vitamin D in their blood. These people were also more likely to suffer from hypertension, coronary artery disease, cardiomyopathy, and diabetes than those with higher levels. Only 29% of the people in the study had the higher level of vitamin D. For details, including the level of vitamin D associated with lower risk of death, ways to boost vitamin D, tests of vitamin D supplements, and more, see the Vitamin D Review >>
CL Answer
10 Supplements That May Help Reduce Nighttime Urination (And 5 That May Not)
Waking up more than once during the night to use the bathroom? Learn more about the 9 supplements that may help and 5 others that don't.

CL Answer
How does turmeric spice compare to curcumin (turmeric extract) in supplements? I sprinkle it on my foods and wonder if that's equivalent to taking a supplement.
What is the difference between turmeric spices used in the kitchen and turmeric supplements? Is sprinkling turmeric on my food equivalent to taking a turmeric supplement? ConsumerLab's answer explores this question.

CL Answer
What are the best supplements for depression and anxiety?
Best natural supplements for depression and anxiety, including the evidence for fish oil, vitamin D, probiotics, and herbal supplements for depression such as curcumin (from turmeric) and saffron.

CL Answer
What are phytoceramides? Do phytoceramide supplements really work to improve aging skin?
Phytoceramide supplement information, including what they are, what they claim to do, and if they have anti-aging effects.

News Release
November 26, 2012
Tests Show Many Milk Thistle Supplements Low in Key Component -- Seven of Eleven Products Fail ConsumerLab.com Testing of Herb Used in Diabetes and Liver Diseases
White Plains, NY, November 26, 2012 — Recent tests by ConsumerLab.com of eleven milk thistle supplements show that only four met ConsumerLab.com's quality standards.
Recalls & Warnings
January 12, 2021
Seller of Cholesterol, Weight Loss Products, and More Warned for Claims
On November 17, 2020, the FDA issued a warning letter to Bonagens following a review of the company's website, which found statements made about the company's products Anti-Aging Supports, Cholesterol & Diabetes Control, Gout Control, Hypertension Control, Liver Supports, Lungs ...
News Release
August 16, 2010
Problems persist with ginseng supplements -- Review by ConsumerLab.com finds 45% of products don't provide full amount of ingredient or are contaminated
WHITE PLAINS, NEW YORK — August 16, 2010 — ConsumerLab.com reported today that five out of eleven ginseng supplements recently selected for testing contained less ginseng than expected from their labels or were contaminated with lead and/or pesticides.
News Release
January 20, 2010
ConsumerLab.com finds quality of most CLA supplements for slimming to be high; One brand mislabeled -- Report is first published in series on supplements for weight loss and slimming
ConsumerLab.com Finds Quality of Most CLA Supplements for Slimming to be High; One Brand Mislabeled
News Release
December 02, 2009
Tests show many milk thistle supplements low in key component -- Nine of ten products fail ConsumerLab.com testing of herb used in diabetes and liver diseases
TESTS SHOW MANY MILK THISTLE SUPPLEMENTS LOW IN KEY COMPONENT
Recalls & Warnings
August 30, 2012
Supplement Company Warned For Medical Claims and Misbranding Of Omega-3, CoQ10, Noni Juice And More
On July 12, 2012, the FDA issued a warning letter to Alfa Vitamins Laboratories, Inc.
Recalls & Warnings
August 08, 2014
FDA Warns Puerto Rico Supplement Seller
On June 30, 2014, the FDA issued a warning letter to Mr. Ramon Rosa, following a review of his website, www.aceitedeguanabana.
Recalls & Warnings
May 30, 2014
Seller of Brain, Diabetes, Echinacea Supplements and More Warned for Drug Claims
On April 22, 2014, the FDA issued a warning letter to Mundo Natural Inc., following a review of the company's website which found statements made about supplements Neuro AD, Stop Diabetes, Stop High Blood Pressure, Morninga 7 and Purpurea Echinacea to be drug claims.
News Release
October 07, 2009
ConsumerLab.com finds that only two-thirds of alpha-lipoic acid supplements selected for testing pass quality review -- Anti-oxidant supplements helpful to diabetics
White Plains, New York — Wednesday, October 7, — Alpha-lipoic acid (also called lipoic acid) is a natural anti-oxidant that can help diabetics reduce symptoms of peripheral neuropathy and control blood sugar, among other uses. ConsumerLab.
Recalls & Warnings
August 25, 2022
White Mulberry Leaf Caused Death of Congressman's Wife, Coroner Reports
The death of Lori McClintock, 61-year-old wife of California congressman Tom McClintock, was caused by dehydration from gastroenteritis caused by “adverse effects of white mulberry leaf ingestion,” according to a coroner's report recently obtained by Kaiser Health News.
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
Recalls & Warnings
June 11, 2015
FDA Warns Maker of Fruit Energy Drinks for Drug Claims
On May 19, 2015, the FDA issued a warning letter to CK Management, Inc. following a facility inspection which found statements made on product labels and websites about Whole5, Fruit of the Spirit and ViaViente "whole food" fruit puree energy drinks to be drug claims.
Recalls & Warnings
October 15, 2007
US Marshalls Seize Charantea Supplements Promoted for Diabetes, Anemia and Hypertension
At the request of the U.S. Food and Drug Administration (FDA), on October 9, 2007, U.S. Marshals seized approximately $71,000 of goods from FulLife Natural Options, Inc., of Boca Raton, Fla., which marketed and distributed Charantea Ampalaya Capsules and Charantea Ampalaya Tea.
Recalls & Warnings
October 30, 2008
Possible Risk Rather than Benefit Found in Trial of Vitamin E and Selenium for Prostate Cancer
On October 27, 2008 the National Cancer Institute (NCI) announced that men in the Selenium and Vitamin E Cancer Prevention Trial (SELECT) are being told to stop taking their supplements.
Recalls & Warnings
November 10, 2020
FDA Warns Immusist for Drug Claims
On October 16, 2020, the FDA issued a warning letter to Immusist, LLC following a review of the company's website, which found statements made about the company's products IMMUSIST Original and IMMUSIST Natural to be drug claims.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
September 08, 2020
Seller of "Diabetes Supplement" & More Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Nutritional Supplements Corporation Inc following a review of the company's website, which found statements made about some of the company's products, including Vitadone, Diabrex, and Viadevita to be drug claims.
Recalls & Warnings
December 17, 2020
FTC Crackdown on Six Deceptive CBD Products
On December 17, 2020, the FTC announced that it is taking action against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, hypertension, Alzheimer's disease, and others.
Recalls & Warnings
November 28, 2014
Seller of Herbal Extracts Warned for Manufacturing Violations, Drug Claims
On November 3, 2014, the FDA issued a warning letter to Avena Botanicals, Inc.
Recalls & Warnings
January 11, 2002
Canada Requests Recall of Certain Ephedra/Ephedrine Products
OTTAWA - January 10, 2002 - Health Canada is requesting a recall from the market of certain products containing Ephedra/ephedrine after a risk assessment concluded that these products pose a serious risk to health.
Recalls & Warnings
March 19, 2004
Seasilver Stopped from Making Claim to Cure "Over 650" Diseases
On March 17, the Food and Drug Administration (FDA) announced that Seasilver USA, Inc., and Americaloe, Inc.
Recalls & Warnings
January 27, 2003
FTC Charges Dr. Clark and Related Companies with Making Unsubstantiated Health Claims
The Federal Trade Commission has charged a Switzerland-based company and its U.S. counterpart with making numerous unsubstantiated efficacy claims for a variety of dietary supplements and devices that they sell on the Internet.
Recalls & Warnings
June 15, 2003
FTC Alleges "Heartbar" Made Deceptive Claims About Effectiveness
On June 12, 2003, the Federal Trade Commission (FTC) announced that Unither Pharma, Inc. and United Therapeutics Corporation have agreed to settle FTC charges that they made deceptive claims in advertising for their HeartBar product.
Recalls & Warnings
July 13, 2006
FDA Warns About Sexual Enhancement Supplements with Dangerous Ingredients
On July 12, 2006, the Federal Drug Administration (FDA) warned consumers not to purchase or consume Zimaxx, Libidus, Neophase, Nasutra, Vigor-25, Actra-Rx, or 4EVERON.
Recalls & Warnings
September 23, 2005
FTC Continues Litigation Against Makers of CortiSlim and CortiStress Supplements
On September 21, 2005 the Federal Trade Commission (FTC) announced that three defendants will give up $4.
Recalls & Warnings
May 28, 2008
FDA Requests Recall of Xiadafil VIP Supplements
On May 27, 2008, the U.S. The U.S. Food and Drug Administration announced that it had requested that SEI Pharmaceuticals, of Miami, Fla.
Recalls & Warnings
July 01, 2005
FDA Issues Nationwide Alert for "Liqiang 4" Due to Potential Health Risk
On July 1, 2005, the U.S. Food and Drug Administration (FDA) warned consumers not to take Liqiang 4 Dietary Supplement Capsules because they contain glyburide – a drug that could have serious, life-threatening consequences in some people.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
February 24, 2015
Seller of Heart, Brain and Diabetes Supplements Warned for Drug Claims
On February 6, 2015, the FDA issued a warning letter to LCW, Inc.
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
April 17, 2012
FDA Warns Seller of Diabetes Supplement Marketed with Rx Claims
The FDA has posted a Warning Letter (dated April 4, 2012) to Leaner Living regarding the marketing of its Glycosolve product.
Recalls & Warnings
November 09, 2023
FDA Warns Biorica International for Cholesterol, Kidney & Cancer Claims
On August 4, 2023, the FDA issued a Warning Letter to Biorica International Corp. following review of the company’s websites and social media, which found statements about the company’s Plaquex Oral Supplements to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
July 15, 2024
Hard Steel Supplements for Men Recalled
On July 12, 2024, Supercore Products Group issued a recall of Hard Steel Capsules and Gold Hard Steel Liquid which are used for male erectile dysfunction. The FDA analysis found the capsules to contain sildenafil and acetaminophen.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
April 02, 2019
FDA Issues Strong Warnings to Sellers of CBD
On March 28, 2019, the FDA issued warning letters to three companies for making unsubstantiated claims about the health benefits of CBD (cannabidiol) products on their websites.
News Release
June 12, 2007
Tests show many milk thistle supplements low in key component — Seven products fail ConsumerLab.com testing of herb used in diabetes and liver diseases
WHITE PLAINS, NEW YORK — June 12, 2007 — Tests by ConsumerLab.com of nine milk thistle supplements that it selected showed that only two contained the expected amounts of silymarin compounds, believed to be the active constituent of milk thistle.
News Release
May 15, 2007
ConsumerLab.com finds some alpha lipoic acid supplements come up short — Improper formulation may cause ingredient degradation
WHITE PLAINS, NEW YORK — May 15, 2007 — ConsumerLab.com announced that two of eleven alpha lipoic acid supplements that it recently chose to test contained significantly less ingredient than promised on their labels.
News Release
May 24, 2006
Problems persist with ginseng supplements — ConsumerLab.com finds nearly half of products lack ingredient or contaminated
WHITE PLAINS, NEW YORK — May 24, 2006 — ConsumerLab.com has released test results for dietary supplements made with ginseng, a popular herb promoted for vitality and the treatment or prevention of a range of medical conditions.
News Release
November 15, 2005
Testing by ConsumerLab.com identifies many problems with popular supplements for weight loss, slimming, and blood sugar control
Westchester, NY — November 15, 2005 — In a series of reports released today, ConsumerLab.com revealed test results for supplements used for weight loss, slimming, and blood sugar control. Among the twenty-three products that ConsumerLab.
News Release
August 31, 2005
ConsumerLab.com testing of alpha lipoic acid supplements from Japan finds no ingredient in one product; Most others meet claims — Japanese consumers urged to research product quality
WHITE PLAINS, NY — ConsumerLab.com reported this week that among nine brands of alpha lipoic acid supplements it recently purchased in Japan and tested, one contained none of the popular ingredient and another contained only 81%.
News Release
December 20, 2004
Testing of Alpha-lipoic acid supplements by ConsumerLab.com finds most meet label claims but one with only 15% of ingredient — Antioxidant of potential benefit in diabetes and other conditions
WHITE PLAINS, NY — December 20, 2004 — ConsumerLab.com announced today that one of 21 alpha-lipoic acid supplements recently tested contained only 15% of the alpha-lipoic acid that it claimed. Alpha-lipoic acid is an antioxidant naturally produced in the body.
News Release
April 03, 2002
More than one in five weight loss, slimming and/or diabetes supplements fail ConsumerLab.com evaluation — Problems found in chromium and pyruvate products; CLA products fine
WHITE PLAINS, NY — April 3, 2002 — ConsumerLab.com announced today that more than one in five products failed its recent Product Review of Weight Loss, Slimming and Diabetes-Management Supplements.
Recalls & Warnings
June 20, 2003
FTC Charges Marketers of Seasilver with False and Deceptive Claims
On June 19, 2003, The Federal Trade Commission (FTC) and the Food and Drug Administration (FDA) announced coordinated actions against two companies - both charged with promoting the dietary supplement "Seasilver" with unsubstantiated medical claims. The agencies' actions against Seasilver USA, Inc.
Recalls & Warnings
August 16, 2006
Marketers of Diabetes Supplement Banned From Claiming Products Treat or Cure Diseases
On August 10, 2006, the Federal Trade Commission (FTC) announced that an operation selling Chinese herbal supplements is banned from claiming its products treat or cure diseases, to settle FTC charges it violated a previous court order.
Recalls & Warnings
October 17, 2012
Herbal Supplement Company Warned For Manufacturing Violations and Drug Claims
On October 2, 2012, the FDA issued a warning letter to Amazing Herbs Nutraceuticals following a facility inspection which found violations of Current Good Manufacturing Practices (CGMPs) for dietary supplements.
Recalls & Warnings
November 06, 2014
Seller of Prostate, Heart Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Health Research Laboratories, LLC/New World Health, following a review of the company's websites, which found statements made about AtheChel Advanced, Betarol, BioTherapex, Omega-3 Cardio Plus, RejuvaLifeRx, and Ultimate Health Formula to be ...
Recalls & Warnings
June 26, 2015
Seller of CoQ10, Anxiety Supplements and More Warned for Manufacturing Violations, Drug Claims
On June 6, 2015, the FDA issued a warning letter to Country Doctor Herbals, following a facility inspection which found the company's products, including Herbal Flu Be-Gone, Herbal Multi-Bac, Herbal Nervine, Herbal Pain-A-Way, Herbal Pancreas, Herbal Parasite Cleanse, Herbal Perfect ...
Recalls & Warnings
December 15, 2020
FDA Warns JC Ayur Life LLC for Drug Claims
On October 29, 2020, the FDA issued a warning letter to JC Ayur Life LLC following a review of the company's website, which found statements made about the company's product Heritage of Ayurveda Dia-Tonic Incudil Herbal Dietary Supplement to be drug claims.
Recalls & Warnings
November 28, 2022
Seller of Joint Health, Collagen Protein and More Warned for Drug Claims
On November 14, 2022, the FDA issued a warning letter to The Truth Company, LLC (parent company of Kinobody, LLC and UMZU, LLC) following inspection of the company’s websites which found statements about its Betaine, Immune, Redwood, Sensolin, Thyrite, zuRelief, Kino Aminos, Kino Collagen ...
Recalls & Warnings
December 12, 2022
FDA Warns Saffron USA for Promoting Teas to Treat Insomnia, Osteoporosis & Cancer
On September 23, 2022, the FDA issued a warning letter to Saffron USA LLC following inspection of the company’s website which found statements about its Allergy Blend, Chamomile Tea Petals, Diabetic Support Blend, Orange Blast Tea, and Saffron Loose Tea products to be drug ...
Recalls & Warnings
November 06, 2014
Seller of Cholesterol, Arthritis Supplements and More Warned for Drug Claims
On October 17, 2014, the FDA issued a warning letter to Vitamins Direct (USA) and Golden Pride, Inc., which distribute Flexezy and Physician's Signature brands of dietary supplements, following a facility inspection which found statements made about certain supplements to be drug claims.
Recalls & Warnings
October 06, 2004
False Claims Made by Marketer of Cortisol-related Weight-Loss Supplements According to FTC
On October 6, 2004, the Federal Trade Commission (FTC)charged marketers of two dietary supplements with claiming, falsely and without substantiation, that their products can cause weight loss and reduce the risk of, or prevent, serious health conditions.
Recalls & Warnings
January 04, 2007
Sellers of Popular Weight Loss Supplements Pay $25 Million Over FTC Allegations of Deceptive Advertising
On January 4, 2007, the Federal Trade Commission (FTC) announced that it had filed complaints in four separate cases alleging that weight-loss and weight-control claims were not supported by competent and reliable scientific evidence.
Recalls & Warnings
May 30, 2020
FTC Sends Refund Checks for TrueAloe and AloeCran
On May 26, 2020, the FTC announced it is mailing 22,581 checks totaling more than $470,000 to consumers who purchased deceptively marketed supplements, TrueAloe and AloeCran.
Recalls & Warnings
February 09, 2022
FDA Warns Seller of Colloidal Silver Eye Drops, Copper Products & More
On February 1, 2022, the FDA issued a warning letter to New Earth Healing Essentials, LLC d/b/a 5D Full Disclosure following a review of the company’s website, which found statements made about some of the company's products, including Plasma Colloidal Silver Eyedrops, Gaia’s ...
Recalls & Warnings
February 17, 2022
Seller of L-Theanine, Lutein, Noopept and More Warned for Drug Claims
On February 4, 2022, the FDA issued a warning letter to Crystal Clear Supplements following a review of the company’s website and social media which found statements about Tianeptine Sodium Powder, Tianeptine Sulfate Powder, PEA Capsules, Phenibut Capsules, Synephrine Powder, Noopept ...
Recalls & Warnings
March 09, 2022
Court Bars Salud Natural From Selling Aloe, Joint Supplements & More
On March 8, 2022, a federal court ordered Salud Natural Entrepreneur, Inc. to stop distributing nutritional supplements that violate the Federal Food, Drug and Cosmetic Act (FDCA).
Recalls & Warnings
August 17, 2022
Seller of Moringa Tea and Other Herbal Products Warned for Drug Claims, Manufacturing Violations
On July 29, 2022, the FDA issued a warning letter to Deggeh Foods, Inc.
Recalls & Warnings
August 25, 2022
Oregon’s Wild Harvest Warned by FDA for Manufacturing Violations
On July 8, 2022, the FDA issued a warning letter to Oregon’s Wild Harvest, Inc.
Recalls & Warnings
May 23, 2013
Maker of Liver Detox and Insulin Supplements Warned For Drug Claims
On April 24, 2013, the FDA issued a warning letter to Glucorell, Inc.
Recalls & Warnings
October 10, 2014
Seller of Multivitamin Warned for Drug Claims
On September 25, 2014, the FDA issued a warning letter to Multimmunity, Inc., following a review of the company's website which found statements made about the dietary supplement Multimmunity to be drug claims.
Recalls & Warnings
May 06, 2014
Seller of Flaxseed, Ginkgo and More Warned for Manufacturing Violations and Drug Claims
On April 18, 2014, the FDA issued a warning letter to Iowa Select Herbs, LLC, following a facility inspection which found the company's products, including Flax Seed, Holy Basil, Papaya Leaf Extract, and Ginkgo Leaf Extract products, to be adulterated because they were prepared, packed, or held ...
Recalls & Warnings
January 26, 2018
Seller of Coenzyme-A Warned For Making Drug Claims
On January 5, 2018, the FDA issued a warning letter to Coenzyme A, Inc. following a facility inspection and review of the company's website that found the company made drug claims about its product Coenzyme-A.
Recalls & Warnings
September 18, 2012
Maker of Noni, Nopal, Blood Sugar and Cholesterol Supplements Warned For Drug Claims, Misbranding, and Manufacturing Violations
On August 27, 2012, the FDA issued a warning letter to Naturavit, Inc. for making statements about dietary supplements Noni Imperial Hawaiian, Garlic and Parsley, Cholestol, Nopal and Diatrin that constitute drug claims.
Recalls & Warnings
September 14, 2016
Seller of Aloe, Prostate and Joint Supplements Warned for Manufacturing Violations
On April 8, 2016, the FDA issued a warning letter to Salud Natural Entrepreneurs, Inc.
Recalls & Warnings
May 30, 2015
Seller of Supplements for Herpes, Prostate Cancer & More Warned for Drug Claims
On May 7, 2015, the FDA issued a warning letter to Strictly Health Corporation, following a review of the company's websites which found statements made about FENVIR, Prosta Pep and Tonalin brand CLA to be drug claims.
Recalls & Warnings
October 18, 2017
Total Body Nutrition Warned for Banned Stimulant in Supplements
On September 28, 2017, the FDA issued a warning letter to Total Body Nutrition following a facility inspection which found some of the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
July 31, 2018
FDA Warns Seller of Joint Health and Cholesterol Supplements
On July 13, 2018, the FDA issued a warning letter to GC Natural, following a facility inspection which found a number of the company's products, including Red Pyrola Plus + Advanced Joint Formula Capsules, CSDP GOLD Capsules, Rejeune Optimal Kidney & Liver Support Extract balls, C.
Recalls & Warnings
September 19, 2018
Seller of Curcumin, Garcinia, Glucosamine and More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to Best Nutrition Products, Inc.
Recalls & Warnings
September 14, 2021
Seller of Lion's Mane, Other Mushroom Supplements Warned by FDA
On July 20, 2021, the FDA issued a warning letter to Brilliant Enterprises LLC because it found statements made on the company's website and social media about mushroom supplements, including Lion's Mane, BoomChaga, CordaCex, and LVL:MAX to be drug claims.
Recalls & Warnings
May 14, 2021
Maker of Care by Design CBD Settles Charges of Unsupported Claims
On May 5, 2021, Cannacraft, Inc.
Recalls & Warnings
May 08, 2021
Seller of Immune Bio Green Cell Warned by FDA
On March 30, 2021, the FDA issued a warning letter to Immune & Genetics Protocols, LLC following a review of the company's websites, which found statements made about the company's product Immune Bio Green Cell to be drug claims.
Recalls & Warnings
April 26, 2021
Family Indicted for Selling Dangerous "Bleach" Miracle Mineral Solution As "COVID" Cure
Four Florida men have been indicted by a Miami federal grand jury on charges of fraudulently marketing and selling the toxic bleach solution Miracle Mineral Solution (MMS) as a cure for COVID-19 and other serious medical conditions, such as cancer, Alzheimer's, diabetes, autism, malaria, ...
Recalls & Warnings
January 20, 2021
Seller of Omega-3 Warned for Making Claims to Treat Focus, Mood
On December 9, 2020, the FDA issued a warning letter to Bodyhealth.com, LLC following a review of the company's website, which found statements made about the company's products Healthy-Thin Energize, Body Detox (Oral Spray), and Omega 3 Health to be drug claims.
Recalls & Warnings
July 21, 2022
Young Living Warned for Promoting Essential Oils to Treat Seasonal Allergies, Kidney Stones & More
On June 10, 2022, the FDA issued a warning letter to Young Living Essential Oils Corporate after an inspection of the company’s website and social media found statements about the company’s essential oil and CBD products to be drug claims.
Recalls & Warnings
April 18, 2022
Moringa Concern: Blood Clots
A 63-year-old woman in New York with type 2 diabetes developed a blood clot in the lungs (i.e. pulmonary embolism) that her physicians suspect may have been caused by the use of a supplement containing Moringa oleifera extract (Ebhohon, Int J Emerg Med 2022).
Recalls & Warnings
December 22, 2021
FDA Warns Seller of Liquid Magnesium, B-12, Berberine & More
On December 9, 2021, the FDA issued a warning letter to Wholly Liquid Nutritional Supplements LLC because it found statements on the company's website and social media about its products, including SpiroLaze, BioLaze, LiquiLaurin, VIT-B12, DeStress, and Omega Plus, to be ...
Recalls & Warnings
March 16, 2006
Garden of Life, Maker of Primal Defense, Settles FTC Charges
On March 9, 2006, The Federal Trade Commission (FTC) announced that an operation that marketed dietary supplements sold at Whole Foods Market, GNC, the Vitamin Shoppe, and on the Internet settled FTC charges that they made deceptive advertising claims about their supplements.
Recalls & Warnings
December 21, 2016
Seller of 5-HTP, Chromium, Curcumin and More Warned for Drug Claims
On December 1, 2016, the FDA issued a warning letter to Aurora Health and Nutrition following a review of the company's website, which found statements made about its products, 5-HTP, Alpha Lipoic Acid, the Argentyn 23 product line, Artecin 90 Vegetarian Capsules, ...
Recalls & Warnings
May 30, 2015
Seller of Omega-3, Probiotics & SuperFoods Warned for Manufacturing Violations, Drug Claims
On May 4, 2015, the FDA issued a warning letter to Dr. Dennis Black, LLC.
Recalls & Warnings
March 10, 2015
Maker of Magnesium, Calcium, Zinc, B Vitamins and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Complete H2O Minerals, following a facility inspection which found the company's products, including Sulfur Concentrate, Copper, Extra Strength Copper, Extra Strength Magnesium, Extra Strength Zinc, Platinum, Gold, Indium, Molybdenum, ...
Recalls & Warnings
September 26, 2014
Sellers of Essential Oils Warned for Claiming to Treat Ebola, Other Diseases
On September 22, 2014, the FDA issued a warning to Young Living following a review of websites, Pinterest, Facebook and Twitter accounts owned by Young Living distributors, which found statements made about the company's essential oils, including Thieves, Cinnamon Bark, Oregano, ImmuPower, ...
Recalls & Warnings
August 02, 2014
Judge Orders BioAnue To Stop Illegal Cancer and Disease Claims
On July 23, 2014, Gloria D.
Recalls & Warnings
September 13, 2013
Metagenics Warned For Misbranding of Medical Foods and Drug Claims
On August 13, 2013, the FDA issued a warning letter to Metagenics, Inc.
Recalls & Warnings
April 26, 2013
Seller of Immune, Cholesterol, Liver and Blood Sugar Products Warned For Drug Claims
On March 20, 2013, the FDA issued a warning letter to Birkdale Medicinals LLC, following a review of the company's website which found statements made about Birkdale products, including Immune Response 247, Cholestat, Silymarin 81 and LevelStat, to be drug claims.
Recalls & Warnings
March 31, 2020
Bulletproof Warned for Manufacturing Violations
On March 20, 2020, the FDA issued a warning letter to Bulletproof 360, Inc.
Recalls & Warnings
March 20, 2020
Seller of Rejuvenation Pills Settles Charges of Making False Claims
Health Center, Inc. has agreed to halt their allegedly deceptive advertising claims about three "cure-all" health and wellness products that targeted older consumers nationwide after the Federal Trade Commission (FTC) filed a complaint.
Recalls & Warnings
November 02, 2019
Brain Supplement Claims to Treat Brain Injuries, Alzheimer's Are Deceptive, Says FTC
On November 1, 2019 the FTC filed a lawsuit against Neora LLC (formerly Nerium International) for making false claims that the supplement Neora EHT (formerly called Nerium EHT) can treat concussions and chronic traumatic encephalopathy caused by repetitive brain trauma, Alzheimer's ...
Recalls & Warnings
June 30, 2020
FDA Warns Seller of Alpha Lipoic Acid, Berberine, and More
On June 18, 2020, the FDA issued a warning letter to Only Natural, Inc. dba Bio Nutrition, Inc.
Recalls & Warnings
May 23, 2013
Seller of Sexual Enhancement, Cholesterol, Resveratrol Supplements and More Warned For Drug Claims
On May 2, 2013, the FDA issued a warning letter to Alternative Health Supplements, following a review of the company's website, which found statements made about several dietary supplements, including Regenerect, Alligin, Astaxanthin Advantage, HDL Cholesterol Management, Resveratrol, Coral Calcium ...
Recalls & Warnings
May 28, 2003
FTC Challenges Claims of Five Supplements to Treat or Cure Serious Diseases
On May 27, 2003 the Federal Trade Commission announced that it had filed a complaint in federal district court against A.
Recalls & Warnings
October 06, 2005
Deceptive Marketing of “Supreme Greens" -- Settlement with FTC
On October 6, 2005, the Federal Trade Commission (FTC) announced that three individuals and two companies have settled FTC charges over their roles in the deceptive marketing of Supreme Greens, an herbal supplement.
Recalls & Warnings
July 13, 2012
Alistrol Health Inc. Warned Over Health Claims on Products
On June 26, 2012, the FDA issued a warning letter to Alistrol Health Inc. for making product statements on the company's websites that constitute drug claims.
Recalls & Warnings
May 17, 2012
FDA Warns of Drug Ingredients in Three Sexual Enhancement Supplements
On May 16, 2012, the U.S. FDA advised consumers not to purchase or use the following three sexual enhancement products: Boost - Ultra Sexual Enhancement Formula (sold on www.boostultra.biz), Firminite (sold on www.firminite.com), and VMaxx Rx (sold on www.vmaxxrx.com).
Recalls & Warnings
May 07, 2012
FDA Warns Doctor Promoting Own Supplements Online as Treatments
On April 18, 2012 the U.S. Food and Drug Administration (FDA) sent a Warning Letter to Jacob Teitelbaum, M.D. of Fatigued to Fantastic, LLC regarding the promotion that company's products on its website www.endfatigue.com.
Recalls & Warnings
April 21, 2012
FDA Finds Prescription Drug Analog in 3 Enhancement Supplements
On April 20, 2012, the U.S. FDA advised consumers not to purchase or use the following supplements from Enhance Nutraceutical: "Instant Hard Rod," "RigiRx Plus," and "Zen Maxx." FDA laboratory analysis confirmed that each contains aminotadalafil.
Recalls & Warnings
April 03, 2012
"France T253" Enhancement Supplement Contains Hidden Drug
April 3, 2012: The U.S. Food and Drug Administration (FDA) is advising consumers not to purchase or use “France T253,” a product for sexual enhancement. This product is promoted and sold on various Web sites, such as ebay.com.
Recalls & Warnings
March 08, 2012
Supplement Maker Warned About Cancer Claims
FDA issued a letter to Royal Domains (dated January 6, 2012) warning that their Nature's Pearl Premium Muscadine Grape Seed dietary supplement is being promoted as a drug and as such is misbranded.
Recalls & Warnings
October 08, 2010
"Red Flags" for Spotting Tainted Supplements
In recent years, the FDA has identified hundereds of products marketed as dietary supplements or conventional foods with hidden drugs and chemicals. In reaction to this, the FDA recently created a flyer to help retailers spot questionable products. ConsumerLab.
Recalls & Warnings
September 13, 2010
Supplement for "Increasing Desire" Recalled for Containing Drug
The U.S. FDA posted a notice regarding the recall of Masxtreme Capsules by the distributor, Natural Wellness, Inc. FDA analysis has determined the product contains undeclared amounts of Aminotadalafil, an analog of tadalafil.
Recalls & Warnings
July 20, 2010
Recall of ED Supplement Containing Drug
On July 20, 2010, the U.S. FDA posted a news announcement from Good Health, Inc. regarding the recall of Vialipro, a sexual enhancement supplement sold nationally.
Recalls & Warnings
April 08, 2010
Capsule for Men Spiked with Erectile Drug
On April 7, 2010, the U.S.
Recalls & Warnings
February 01, 2012
FDA Warns Seller of Almased Weight Loss Product of Marketing Violations
On January 18, 2011, the U.S. FDA sent a Warning Letter to Almased USA regarding legal violations in its marketing of its Almased product (Almased Synergy Diet).
Recalls & Warnings
July 23, 2009
Six Male Enhancement Supplements Found Adulterated
On July 15, 2009, the U.S. FDA announced that Obteron 1 Inc. dba Nature & Health Co. is recalling the following supplements: LibieXtreme, Y-4ever, Libimax X Liquid, Powermania Capsule, Powermania Liquid, and Herbal Disiac.
Recalls & Warnings
March 08, 2016
Maker of Calcium and Vitamin C Supplements Warned for Manufacturing Violations
On September 17, 2015, the FDA issued a warning letter to Raphah, Inc.
Recalls & Warnings
June 04, 2004
FTC Charges Marketers of Two Supplements with False Claims to Cure Range of Diseases
On June 3, 2004 the Federal Trade Commission (FTC) charged marketers of two dietary supplements with falsely claiming that their products can prevent and cure cancer and other diseases. According to the FTC’s complaint, Boston-area marketers Direct Marketing Concepts, Inc. (DMC), ITV Direct, Inc.
Recalls & Warnings
December 10, 2002
Company Ordered to Cease Deceptive Marketing of Weight Loss Product
A U.S. District Court in Texas has ordered Mark Nutritionals, maker of Body Solutions Evening Weight Loss Formula, to stop making certain claims with regard to the product. The order is in response to a complaint by the Federal Trade Commission (FTC) accusing the company of false advertising.
Recalls & Warnings
December 12, 2018
Seller of Protein Powder, Spirulina & Other Products Warned for Manufacturing Violations
On October 31, 2018, the FDA issued a warning letter to DynaPro International, Inc.
Recalls & Warnings
December 01, 2018
Seller of 5-HTP, Potassium & More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to The Delano Company, Inc.
Recalls & Warnings
October 22, 2019
Seller of TrueAloe and AloeCran Settles Charges of Making False Claims
NatureCity, LLC, has agreed to settle Federal Trade Commission (FTC) charges that they deceived consumers by making false claims about their TrueAloe and AloeCran products.
Recalls & Warnings
April 09, 2003
Recall of Dangerous Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On April 4, 2003, the U.S. Food and Drug Administration's MedWatch program announced that Ultra Health Laboratories, Inc. and Bionate International, Inc. are warning consumers not to purchase or consume a product known as Vinarol tablets.
Recalls & Warnings
November 03, 2004
FDA Warns of Sexual Enhancement Supplements Containing Prescription Drug
On November 2, 2004, the Food and Drug Administration (FDA)warned consumers not to purchase or to consume Actra-Rx or Yilishen, two products promoted and offered for sale on Web sites as "dietary supplements" for treating erectile dysfunction and enhancing sexual performance for men.
Recalls & Warnings
June 23, 2003
FDA Warns Consumers Against Taking 6 Sexual Enhancement Supplements
On June 20, 2003, the Food and Drug Administration (FDA) warned consumers not to purchase or consume the following products: SIGRA, STAMINA Rx and STAMINA Rx for Women, Y-Y, Spontane ES and Uroprin, manufactured by NVE, Inc., in Newton, N.J. and distributed by Hi-Tech in Norcross, Ga.
Recalls & Warnings
May 29, 2003
Recall and Warning for Another Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On May 23, Best Life International, in cooperation with the U.S. Food and Drug Administration, warned consumers not to purchase or consume the product known as Viga.
Recalls & Warnings
March 06, 2009
"The Truth About Nutrition" Marketers Agree to Pay $3 Million to Settle Charges of Deceptive Advertising of Dietary Supplements and Devices
On March 6, 2008, the Federal Trade Commission (FTC) announced that marketers of dietary supplements and health-related devices have agreed to pay $3 million in consumer redress to settle FTC charges that they deceptively claimed their products treated or prevented a wide variety of serious ...
Recalls & Warnings
March 20, 2007
Recall of Adulterated Sexual Enhancement Supplement
As posted on the FDA website, on March 15, 2007, the company Barodon SF of Los Angeles, CA announced that it is conducting a voluntary nationwide recall of the company's supplement product sold under the name V.MAX.
Recalls & Warnings
November 26, 2007
Encore Tabs Recalled -- Contain Prescription Drug
On November 21, 2007, Bodee LLC announced today that it is conducting a voluntary nationwide recall of all the company's supplement product sold under the name Encore Tabs.
Recalls & Warnings
November 29, 2016
Seller of Mineral, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On July 1, 2016, the FDA issued a warning letter to BioGenyx-Basic Reset, following a facility inspection which found the company's products, including Ionyte, Aqualyte, Beta Factor, Bee Gold and Primo Java to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
March 13, 2018
FDA Warns Seller of Milk Thistle, Chromium, Joint Supplements & More for Manufacturing Violations
On March 9, 2018, the FDA issued a warning letter to Carol Bond Health Foods following a facility inspection which found a number of the company's products, including Dolomite, Lipo Fat Fighter, ThistleX, Injuv, and Vinpocentine, to be ...
Recalls & Warnings
February 02, 2018
Seller of Vitamin D, Omega-3's, Whey Protein & More Warned for Drug Claims
On January 24, 2018 the FDA issued a warning letter to Young Health Products, LLC following a review of the company's website which found promotional statements and testimonials made about the certain products, including Lugol's Solution 2%, Liquid Vitamin D3, Probimune, Flax Seed & Omega ...
Recalls & Warnings
August 01, 2017
"Man of Steel" Sexual Enhancement Supplements Recalled
On July 27, 2017, Man of Steel recalled its Man of Steel 1 and Man of Steel 2 sexual enhancement supplements because they were found to contain undeclared sildenafil.
Recalls & Warnings
June 27, 2017
Seller of Alpha Lipoic Acid, Cinnamon Supplements and More Warned For Manufacturing Violations
On May 1, 2017 the FDA issued a warning letter to Nature's Vision, Inc.
Recalls & Warnings
April 08, 2017
Woman Dies After Receiving Turmeric Infusion
On March 16, 2017, a San Diego, California woman died days after receiving an IV infusion of turmeric. Jade Erick, age 30, suffered a brain injury and cardiac arrest after receiving the first 5 mL of a 250 mL infusion of turmeric in the office of a naturopathic doctor.
Recalls & Warnings
September 19, 2018
FDA Warns Seller of Shilajit
On September 6, 2018, the FDA issued a warning letter to Adaptive Energy LLC, following an inspection of the company's website, which found statements made about its shilajit product, Purblack were found to be drug claims.
Recalls & Warnings
February 06, 2016
Seller of Turmeric, Milk Thistle and More Warned for Manufacturing Violations, Drug Claims
On January 15, 2016, the FDA issued a warning letter to Terra Firma Botanicals, Inc.
Recalls & Warnings
January 26, 2016
Seller of CoQ10, SAM-e, Vitamin D, and More Warned for Manufacturing Violations, Drug Claims
On January 15, 2016, the FDA issued a warning letter to Nutri-Dyn Midwest, Inc., following a facility inspection which found the company's products, including Cardioauxin BP, Zinc Lozenge, Oliver, Pau D'Arco, Petadolex, Chondro Jointaide, Dynagesic, SAMe-200, Gugulipid.
Recalls & Warnings
January 20, 2016
Maker of Growth Hormone, Testosterone Booster Warned for Manufacturing Violations, Misbranding
On January 8, 2016, the FDA issued a warning letter to Nutraloid Labs Inc.
Recalls & Warnings
January 20, 2016
Seller of Aloe, Moringa Supplements Warned for Manufacturing Violations, Drug Claims
On January 13, 2016, the FDA issued a warning letter to Alkebulan International Services, LLC, following a facility inspection which found the company's products,Aloe Ferox and Moringa Oleifera Capsule to be adulterated because they prepared, packed, or held under conditions that ...
Recalls & Warnings
December 29, 2015
Maker of Vitamin K, Vitamin A & More Warned for Manufacturing Violations, Drug Claims
On December 10 2015, the FDA issued a warning letter to Dherbs Health Emporium, Inc.
Recalls & Warnings
September 07, 2016
Seller of Herbal Formulas for Cholesterol, Prostate & More Warned For Drug Claims
On July 29, 2016, the FDA issued a warning letter to Healing-Scents following a review of the company's website, which found statements made about its products, Heart Herbs, Cholesterol Regulation Herbs, Diabetes Regulation Herbs, Prostate Healer Herbs, High Blood Pressure Herbs, ...
Recalls & Warnings
August 02, 2016
Seller of Whey Protein Warned for Manufacturing Violations, Drug Claims
On July 22, 2016, the FDA issued a warning letter to New Horizon Nutraceuticals, LLC, following a facility inspection which found the company's product, One World Whey Protein Power Food to be adulterated because it was prepared, packed, or held under conditions that violate Current ...
Recalls & Warnings
July 23, 2016
Maker of "Super Food" Warned for Manufacturing Violations
On July 12, 2016, the FDA issued a warning letter to TerraVare, Inc.
Recalls & Warnings
May 24, 2016
Seller of Joint Health, Omega-3 Supplements & More Warned for Manufacturing Violations, Drug Claims
On May 13, 2016, the FDA issued a warning letter to Rocky Fork Formulas, Inc.
Recalls & Warnings
March 29, 2016
Maker of Bone Health, Prostate, Cholesterol Supplements and More Warned for Drug Claims
On March 17, 2016, the FDA issued a warning letter to Rx Vitamins, Inc., following a facility inspection which found the company's products, includingTestost-Rx, DB-7, The Bone Density Formula, NaturLo Cholesterol, ThyRx-7 and Arth-9 to be misbranded.
Recalls & Warnings
March 08, 2016
Seller of Echinacea Warned Manufacturing Violations, Drug Claims
On February 25, 2016, the FDA issued a warning letter to Herbal Energetics/ In Joy Organics, following a facility inspection which found the company's product, X Out-Rays to be adulterated because it was prepared, packed, or held under conditions that violate Current Good ...
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
November 18, 2015
Seller of "Hangover" Supplement Warned for Manufacturing Violations & Drug Claims
On September 17, 2015, the FDA issued a warning letter to Life Support Development Ltd, following a facility inspection which found the company's product, including Life Support Hangover Relief to be adulterated because they prepared, packed, or held under conditions that violate ...
Recalls & Warnings
September 26, 2015
Seller of Muscle Supplements and More Warned for Manufacturing Violations
On August 14, 2015, the FDA issued a warning letter to Chaotic Labz, Inc.
Recalls & Warnings
September 05, 2015
Maker of Multivitamin and Fish Oil Warned for Manufacturing Violations
On July 22, 2015, the FDA issued a warning letter to Westar Nutritional Corp. dba Viva Life Science, Inc.
Recalls & Warnings
August 23, 2015
Maker of Herbal Supplements Sold on eBay, Amazon and Other Websites Shut Down
On August 17, 2015, a federal court ordered a permanent injunction against dietary supplement company Iowa Select Herbs LLC for unlawfully manufacturing and distributing unapproved new drugs and misbranded drugs and adulterated and misbranded dietary supplements.
Recalls & Warnings
August 11, 2015
Company Ordered to Stop Making and Selling Supplements
On August 4, 2015, a federal court ordered a permanent injunction against dietary supplement company Atrium Inc., and two companies under the same ownerhship, Aspen Group Inc., Nutri-Pak of Wisconsin Inc.
Recalls & Warnings
July 02, 2015
Maker of Arthritis Supplement Warned for Manufacturing Violations, Drug Claims
On June 17, 2015, the FDA issued a warning letter to Desert Stream, Inc.
Recalls & Warnings
July 01, 2015
Undeclared Drugs Found In Male Enhancement Supplements
On June 10, 2015, the FDA issued a warning letter American Lifestyle, following tests which found the company's sexual enhancement supplements Vicerex and Sudibil-Xr to contain the undeclared drugs propoxyphenyl thioaildenafil and tadalafil.
Recalls & Warnings
March 10, 2015
Seller of Vision Supplements Warned for Manufacturing Violations, Drug Claims
On February 26, 2015, the FDA issued a warning letter to Biosyntrx, following a facility inspection which found the company's products, including BioTears Oral Gel Caps, ZoOmega-3 Concentrated Pharmaceutical-Grade Fish Oil, EpiCor A Nutrient-dense High Metabolite Immunogen, Sight C+ Mineral ...
Recalls & Warnings
March 06, 2015
Seller of Tea Drinks Warned for Drug Claims
On February 26, 2015, the FDA issued a warning letter to the seller of Anamu CancerHerb Tea Box, Anamu CancerHerb Tea Box with Honey and Organic Bottled Tea Drinks because statements made about these products on the websites, www.cancerherbtea.com and www.anamucancerherbtea.
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
May 23, 2015
Seller of Magnesium, Calcium, Silver and More Warned for Manufacturing Violations, Drug Claims
On May 8, 2015, the FDA issued a warning letter to Pick and Pay, Inc.
Recalls & Warnings
May 02, 2015
Maker of Garcinia, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On March 25, 2015, the FDA issued a warning letter to JW Nutritional LLC, following a facility inspection which found the company's products, including Vanish V2 Domestic, Mr.
Recalls & Warnings
March 17, 2015
Seller of Tea Warned for Drug Claims
On March 4, 2015, the FDA issued a warning letter to Four Elements Organic Herbals, LLC because statements made about HERBAL TEA Power, Energy & Stamina, ORGANIC HERBAL TEA Tulsi TelepaTea, ORGANIC HERBAL TEA To Your Health, Hawthorn Fresh Herb Extract, Elderberry Fresh Herb ...
Recalls & Warnings
February 10, 2015
Seller of "Natural" Cough Syrup Warned for Manufacturing Violations, Drug Claims
On January 21, 2015, the FDA issued a warning letter to Fragrance Manufacturing Incorporated, following a facility inspection which found the company's products, including Maty's All Natural Cough Syrup and Maty's All Natural Cough Syrup for Kids, to be adulterated because they ...
Recalls & Warnings
January 31, 2015
Maker of Soy and Zinc Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to PreMark Health Science, Inc.
Recalls & Warnings
January 27, 2015
Seller of Aloe, Sexual Enhancement Supplements and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Aloe Man, Inc., following a facility inspection which found the company's products, including The Aloe Man's Super Bright, Dr. Johnson's Maximum Desire, Dr. Johnson's Body Healer, and Dr.
Recalls & Warnings
January 23, 2015
Maker of Vitamin Drinks Warned for Manufacturing Violations
On January 7, 2015, the FDA issued a warning letter to NYSW Beverage Brands, Inc.
Recalls & Warnings
January 21, 2015
Supplement Maker Ordered to Stop Selling Products
On January 15, 2015, a federal judge ordered a permanent injunction against dietary supplement manufacturer Health One Pharmaceuticals, Inc. which requires the company to stop manufacturing and selling dietary supplements.
Recalls & Warnings
January 17, 2015
Supplement Maker Warned for Manufacturing Violations
On August 5, 2014, the FDA issued a warning letter to Engineering Nutrition, following a facility inspection which found the company's products, which were not named, to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for ...
Recalls & Warnings
January 17, 2015
Maker of Magnesium and Potassium Supplements Warned for Manufacturing Violations
On December 23, 2014, the FDA issued a warning letter to Nutri Spec, Inc.
Recalls & Warnings
December 30, 2014
Maker of Probiotic Supplement Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to Wellmill LLC, dba Vitamix, following a facility inspection which found the company's products, including Butcher's Broom powder and Lactobacillus acidophilus powder (used as ingredients in a finished product which was not named) to be ...
Recalls & Warnings
December 30, 2014
Maker of Aloe, Weight Loss Supplements and More Warned for Manufacturing Violations, Drug Claims
On December 17, 2014, the FDA issued a warning letter to Dandy Day Corporation, following a facility inspection which found the company's products, including Aloe Pearl, Alfalo, Aloespring, Crave Away, Original Supreme Energy, Ener "G," Can G, Flora G, Flora G Plus, and Garolic, to be adulterated ...
Recalls & Warnings
December 12, 2014
Seller of Omega-3 "Syrup" Warned for Manufacturing Violations and Drug Claims
On October 16, 2014, the FDA issued a warning letter to Mr.
Recalls & Warnings
April 07, 2010
Nationwide Recall of Masxtreme Capsules Containing Drugs with Cardiovascular Side Effects
On March 30, 2010, the U.S. Food and Drug Administration (FDA) posted a notice from Natural Wellness warning consumers not to purchase or consume the product known as MasXtreme, Lot# 911035.
Recalls & Warnings
January 31, 2010
Glucomannan May Cause Choking if Not Taken with Fluid
On January 29, 2010, the Canadian health department, Health Canada, adised that natural health products containing the ingredient glucomannan in tablet, capsule or powder form have a potential for harm if taken without at least 8 ounces of water or other fluid.
Recalls & Warnings
December 14, 2009
Nationwide Recall of Sexual Enhancement Supplements Containing Drug-like Compound
On December 14, 2009, the Federal Drug Administration (FDA) posted a notice on its website regarding the recall of many sexual enhancement supplements sold by Atlas Operations, Inc.
Recalls & Warnings
July 30, 2008
Recall of Viapro Capsules Due to Potentially Harmful Ingredient
The U.S. FDA has posted a release noting that EG Labs, LLC announced a nationwide voluntary recall on July 23, 2008 of all lots of its supplement product sold under the brand name, Viapro, in 375mg capsules.
Recalls & Warnings
May 29, 2008
Recall of Virility Supplement
On May 29, 2008, the FDA posted a recall by International Pharmaceuticals, Ltd. of all of the company's supplement sold under the name of Viril-Ity-Power (VIP) Tabs, 560mg/serving.
Recalls & Warnings
March 04, 2008
Two Supplements Recalled for Containing Viagra-like Compounds
On March 4, 2008, the FDA posted a release from Palo Alto Labs (dated February 28) announcing a voluntary nationwide recall of the company's supplement products sold under the name Aspire36 and Aspire Lite.
Recalls & Warnings
September 12, 2010
Mr. Magic Male Enhancer Recalled
The U.S. FDA posted a recall announcement daated August 18, 2010 from Glow Industries regarding "Mr. Magic Male Enhancer from Don Wands.
Recalls & Warnings
August 13, 2010
Recall of Male Enhancement Supplement Sold in National Stores
On August 9, 2010 the U.S. FDA announced that the company Prolatis' of Salt Lake City, Utah is conducting a voluntary recall of the company's product sold as Prolatis'.
Recalls & Warnings
July 19, 2011
Recall of Enhancement Supplement for Men - Contains Drug Compounds
On July 13, 2011, Global Wellness, LLC announced an expanded voluntary nationwide recall of Via Extreme Ultimate Sexual Enhancer Dietary Supplement for Men. The product was distributed throughout the U.S. Puerto Rico, Barbados, and Canada to internet and retail consumers.
Recalls & Warnings
June 07, 2011
FDA Seizes Elderberry Juice Concentrate Due to Unproven Claims
On June 3, 2011, The U.S. FDA announced that, at its request, U.S. Marshals seized elderberry juice products that have been distributed by Wyldewood Cellars Inc., based in Peck, Kan., because the products are unapproved and misbranded drugs.
Recalls & Warnings
February 24, 2011
Recall of Counterfeit Extenze Tablets Containing Drugs
On February 23, 2011, the FDA announced that Biotab Nutraceuticals, Inc. was notified that two lots of counterfeit product purporting to be Extenze contain undeclared drug ingredients. Specifically, lot 0709241 contains tadalafil and sildenafil, and lot 0509075 contains tadalafil and sibutramine.
Recalls & Warnings
January 01, 2011
Recall of Two Supplements Spiked with Erectile Dysfunction Drug
On December 22, 2010 the U.S.
Recalls & Warnings
March 03, 2012
Seller of Cancer Cures Warned by FDA
On February 9, 2012, the U.S. FDA sent a Warning Letter to BioAnue Laboratories, Inc. informing it that its websites at www.tumorx.com, www.cancerx.org, www.hopewelltechnologieslimited.com, and www.vmhe.
Recalls & Warnings
March 02, 2012
Recall of Enhancement Supplement for Men -- Contains Drug
On Feb, 24, 2012, Regeneca, Inc. announced a voluntary nationwide recall of all lots of RegenErect. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a blue capsule sold individually in foil packets with a UPC code of 816860010055.
Recalls & Warnings
February 16, 2012
Recall of Enhancement Supplement for Women -- Contains Drug
On Feb, 10, 2012, Regeneca, Inc. announced a voluntary nationwide recall of RegenArouse Natural Female Intimacy Enhancement. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a pink capsule sold individually in foil packets.
Recalls & Warnings
February 07, 2012
Drug-Laced Supplements and Drink Mixes Recalled
According to the U.S. FDA, on February 3, 2012 Healthy People Co. announced a voluntary nationwide recall of several dietary supplements sold as capusules, drinks, and shake mixes. The supplements were found to contain drug substances and pose risks.
Recalls & Warnings
March 08, 2012
Rx Drugs Found in Weight Loss and Enhancement Supplements
On February 6, 2012, FDA issued a letter to Globe All Wellness, LLC warning that two of their products contain undeclared prescription drugs.
Recalls & Warnings
May 29, 2013
Seller of Colloidal Silver, Noni Juice and Noni Supplements Warned For Drug Claims
On May 16, 2013, the FDA issued a warning letter to Matrix Health Products, Inc.
Recalls & Warnings
January 30, 2013
Seller of Omega-3 and Emu Oil Supplements Warned For Drug Claims, Misbranding
On November 19, 2012, the FDA issued a warning letter to Emu Products & Management, Inc. after a review of the company's website found statements that promoted the dietary supplements Multi-Omega Gel Caps, Recovery Gel Caps, ARP Gel Caps and the topical skin products Extreme Cryo Gel, F.A.C.E.
Recalls & Warnings
July 26, 2014
Marketers of Nopal Cactus Drink Settle FTC Charges of Deceptive Claims
TriVita Inc., and marketers of the company's "prickly pear" fruit drink Nopalea have agreed to pay $3.5 million in consumer refunds to settle FTC charges they made deceptive claims that the drink treats health problems ranging from skin conditions to joint pain and respiratory problems.
Recalls & Warnings
July 24, 2014
Seller of Noni Juice Products Warned for Manufacturing Violations, Drug Claims
On July 14, 2014, the FDA issued a warning to Noni Connection dba Puna Noni, following a facility inspection which found the company's Puna Noni 100% Pure Hawaiian Noni Fruit Capsules to be adulterated because they were prepared, packed, or held under conditions that violate Current Good ...
Recalls & Warnings
July 11, 2014
Maker of Cough and Sleep "Remedies" Warned for Drug Claims
On June 27, 2014, the FDA issued a warning letter to Zarbee's, Inc.
Recalls & Warnings
January 09, 2014
Marketers of "Genetically Customized" Supplements Settle FTC Charges of Deceptive Health Claims
Two marketers of "genetically customized" nutritional supplements have agreed to a settlement with the Federal Trade Commission (FTC) over charges that the companies made deceptive advertising claims and did not adequately protect customers' medical and financial information.
Recalls & Warnings
December 05, 2014
Maker of Ginseng, Zinc, Calcium and More Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Long Island Pharmaceuticals, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
November 17, 2014
Probiotic Powder Recalled
On November 14, 2014, Solgar, Inc. issued a voluntary recall of Solgar ABC Dophilus Powder (NET Wt. 1.75 oz (50 g)) because it was found to contain Rhizopus oryzae, a fungus which may cause Mucormycosis infection.
Recalls & Warnings
June 03, 2015
Maker of B-12 Energy Supplement Warned for Manufacturing Violations
On May 15, 2015, the FDA issued a warning letter to LiquidCapsule Manufacturing, LLC.
Recalls & Warnings
November 25, 2007
Recall of Men's "Sexual Energy" Supplement Expanded to Include "Energy Max" Product
On November 16, 2007, American True Man Health Incorporated announced that it is expanding it's voluntary recall of the Company's dietary supplement product sold under the name and identified as True Man's Sexual Energy Nutriment Men's Formula with the expiration date up to and including December ...
Recalls & Warnings
May 10, 2007
FDA Warns of Two Supplements Containing Pharmaceutical-like Compounds
On May 10, 2007, the FDA advised consumers not to purchase or use "True Man" or "Energy Max" products promoted and sold as dietary supplements throughout the United States.
Recalls & Warnings
April 19, 2007
Recall of Another Sex Enhancement Supplement
On April 17, 2007 the Food and Drug Administration (FDA) website posted an announcement that Jen-On Herbal Science International, Inc. is conducting a voluntary nationwide recall of the company's supplement product sold under the name H S Joy of Love. Jen-On Herbal Science International, Inc.
Recalls & Warnings
October 12, 2005
Supplement Company to Pay $2 Million Penalty For Alleged Violations of FTC Order
On October 12, 2005 the Federal Trade Commission (FTC) announced that under the terms of a consent decree approved by the it for submission by the U.S. Department of Justice (DOJ) to the federal court for approval, NBTY, Inc. (NBTY, formerly Nature’s Bounty, Inc.
Recalls & Warnings
October 10, 2003
Government Stops Sale of Supplements Claiming to Treat Obesity and Impotence
On October 8, 2003, The Food and Drug Administration (FDA) announced that on September 22, 2003, U.S. District Court Judge Robert J. Vining, Jr.
Recalls & Warnings
February 24, 2015
Seller of Antioxidant Water, Energy Drops Warned for Manufacturing Violations and Drug Claims
On February 18, 2015, the FDA issued a warning letter to Better Health Lab, Inc.
Recalls & Warnings
March 29, 2013
Prescription Drugs Found In Prostate and Sexual Enhancement Supplements
October 24, 2012 the FDA issued a warning letter to USA Far Ocean Group Inc./ Health & Beauty Group Inc. because a number of the company's dietary supplements were found to contain pharmaceutical drugs, or were promoted with statements that constitute drug claims.
Recalls & Warnings
February 13, 2013
Manufacturer of Heart, Multivitamin, Cranberry, and Longevity Supplements Warned For Adulteration and Drug Claims
On January 29, 2013, the FDA issued a warning letter to M.D.R. Fitness Corp. following a facility inspection which found violations of good manufacturing practices which cause the company's products to be adulterated.
Recalls & Warnings
May 10, 2013
Maker of Concentration, Pain, Immune, Cholesterol Supplements Warned For Drug Claims
On April 24, 2013, the FDA issued a warning letter to EuroPharma Co., Inc., following a review of the company's website which found statements made about Calm Kids, CholestCaps, CuraMed 375 mg, CuraMed 750 mg, Curamin, Mental Advantage, Tri-Iodine, and Viragen to be drug claims.
Recalls & Warnings
May 03, 2013
Maker of Omega-3, Saw Palmetto, St. John’s Wort Supplements And More Warned For Manufacturing Violations and Drug Claims
On March 19, 2013, the FDA issued a warning letter to Sunset Natural Products Inc.
Recalls & Warnings
June 07, 2013
Seller of Red Rice Yeast With CoQ10, Respiratory Supplement And More Warned For Manufacturing Violations and Drug Claims
On February 25, 2013, the FDA issued a warning letter to Altasource, LLC, dba Meta Labs LLC, following a facility inspection which found the company's dietary supplements, including Respiratory Response, African Mango, Coffee Black Salve, Meta-Cell, Conjugated Linoleic Acid and Red Yeast Rice with ...
Recalls & Warnings
December 30, 2007
FDA Warns Consumers Not to Use Several "Shangai" Sexual Enhancement Supplements
On December 28, 2007, the U.S. Food and Drug Administration (FDA) advised consumers not to buy or use Super Shangai, Strong Testis, Shangai Ultra, Shangai Ultra X, Lady Shangai, and Shangai Regular, also marketed as Shangai Chaojimengnan, products.
Recalls & Warnings
March 25, 2008
FDA Warns of Sexual Supplements with Drug-like Ingredients
On March 25, 2008, the U.S. Food and Drug Administration advised consumers not to purchase or use "Blue Steel" or "Hero" products marketed as dietary supplements throughout the United States because they are considered unapproved drugs and have not been proven to be safe or effective.
Recalls & Warnings
July 29, 2008
Recall of Sexual Enhancement Supplements Containing Drug
On July 28, 2008 the U.S. FDA announced that Jack Distribution, LLC and G & N works, Inc. are conducting a voluntary nationwide recall of all lot numbers of the company's supplement products sold under the brand names Rize 2 The Occasion and Rose 4 Her.
Recalls & Warnings
July 28, 2008
Seizure of Xiadafil VIP Tablets After Company Refuses Recall
On July 24, 2008, the FDA announced that U.S. Marshals seized nearly $74,000 worth of Xiadafil VIP tablets, Lots 6K029 and 6K209-SEI, distributed by SEI Pharmaceuticals, Inc. of Miami, Fla.
Recalls & Warnings
August 31, 2009
Court Orders Marketers of Supreme Greens and Coral Calcium to Pay Nearly $70 Million for Consumer Refunds
On August 27, 2009, a federal district court ordered the marketers of two dietary supplements – "Supreme Greens" and "Coral Calcium" – who claimed the products would cure ailments ranging from cancer and Parkinson’s disease to heart disease and autoimmune diseases to pay nearly $70 million for ...
Recalls & Warnings
June 15, 2009
Sexual Enhancement Supplement Recalled -- Second Time Found with Drug-like Compound
On June 15, 2009, the U.S. FDA announced that, per its order, Hi-Tech Pharmaceuticals, Inc. is conducting a nationwide voluntary recall of the company's product sold under the name Stamina-Rx.
Recalls & Warnings
September 09, 2011
Possible Concern with Fenugreek Based on Contamination in Europe
Products made with fenugreek, including pills containing fenugreek seed, have been pulled from shelves in Germany due to conern over potential contamination with E. coli bacteria. There is no such action in the U.S., but ConsumerLab.
Recalls & Warnings
December 12, 2014
Seller of B-12, Zinc, Echinacea and More Warned for Manufacturing Violations and Drug Claims
On November 14, 2014, the FDA issued a warning letter to Scientific Botanicals Company, Inc.
Recalls & Warnings
November 07, 2015
Seller of "NaturalDoctor" Vitamin C, Echinacea and More Warned for Manufacturing Violations
On October 16, 2015, the FDA issued a warning letter Sound Healing Arts, PC, dba Grounds for Tea, LLC, following a facility inspection which found the company's products, including NaturalDoctor Vitamins C & K3, NaturalDoctor Goldenseal & Echinacea Plus, NaturalDoctor Centella ...
Recalls & Warnings
December 16, 2015
Maker of Red Yeast Rice, St. John's Wort, Valerian and More Warned for Manufacturing Violations, Drug Claims
On December 2, 2015, the FDA issued a warning letter to Nature's Health, LLC, following a facility inspection which found the company's product, including Ginkgo & Rhodiola, Blood Sugar Balance IV, Cinnamon Extract, Choles-Balance Red Yeast Extract, Lecithin, Milk Thistle Seed Extract, ...
Recalls & Warnings
September 12, 2018
FDA Urges Consumers to Avoid Kratom
On September 11, 2018, FDA Commissioner Scott Gottlieb, M.D., urged consumers not to use products containing the kratom, an herb that is often promoted for pain relief and for relieving symptoms of opioid withdrawal.





