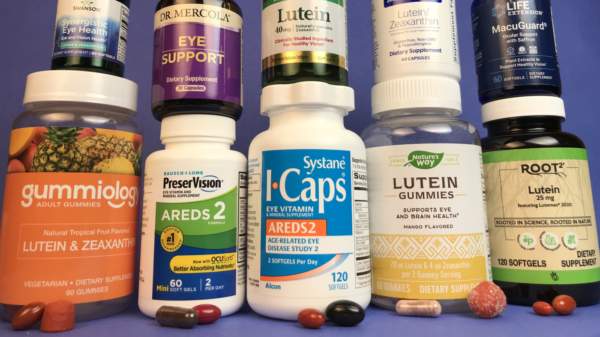
Reviews and Information for Eye Health Supplements
Vision Supplements with Lutein, Zeaxanthin & AREDS2 Formulas
Find the best vision supplements containing lutein and zeaxanthin. See tests of popular brands like PreserVision, ICaps, MacuGuard, MacuGuard, and more. Find out which supplements most closely resemble AREDS and AREDS2 formulas and see how products compare. Review the evidence for slowing the progression of age-related macular degeneration.
Vision Supplements with Lutein, Zeaxanthin & AREDS2 Formulas ReviewSearch term may appear only in full report available to members. Join now for full access.
News Release
October 30, 2025
ConsumerLab.com Reveals Key Safety Tips for Cinnamon Users: What You Need to Know About Toxic Compounds and Health Benefits
October 30, 2025 — Cinnamon is a popular spice and supplement, but new testing by ConsumerLab.com highlights important safety considerations for regular users. Dr. Tod Cooperman, president of ConsumerLab.
Product Review
Zinc Supplements, Lozenges, and Melts Review
Find the Best Zinc Supplements, Including Lozenges for Colds.

Product Review
Vitamin E Supplements Review
Find the Best Vitamin E Supplement. Tests and Reviews of Popular Vitamin E Supplements & CL's Top Picks.
CL Answer
Which supplements are helpful for age-related macular degeneration (AMD)?
Find out which vitamins & supplements are helpful for macular degeneration (AMD), such as lutein, zeaxanthin, and others used in the AREDS studies.

Product Review
Digestive Enzyme Supplements Review
Big Differences in Strength Found Among Digestive Enzyme Supplements. CL Tests Reveal Which Are Best.

CL Answer
Do any supplements help prevent or improve cataracts?
Find out which supplements may help with cataracts, including selenium and vitamins A, B1, B3, C, and E.

CL Answer
Can supplements, natural eye drops, or lifestyle modifications improve symptoms of dry eye?
Can supplements such as fish oil, flaxseed oil or curcumin (from turmeric) help treat dry eyes? Find out, including formulas and doses that have shown benefit. Also, learn if drinking more water or using eye drops containing castor oil, N-acetylcarnosine or aloe can help with dry eye.

CL Answer
What is lactoferrin and will it really strengthen my immune system?
Info about lactoferrin supplements, clinical evidence for strengthening the immune system, preventing or treating colds, gut health and treating H.
Product Review
Lavender and Tea Tree Essential Oils Review
Oils Tested for Authenticity and Purity

CL Answer
What are the side effects of glucosamine and chondroitin?
Learn the side effects of glucosamine and chondroitin supplements for joint health and joint pain, including stomach upset, headache, and many others. ConsumerLab.com's answer explains.

Product Review
Manuka Honey Review
Find the Best Manuka Honey Based on Quality, Taste, and Value. See our Top Picks.

CL Answer
Does glucosamine raise "bad" LDL cholesterol?
Learn more about the link between glucosamine and cholesterol, including evidence from clinical studies.

CL Answer
Does red and near infrared light therapy reduce pain, improve skin or cognition, or have other benefits? Is it safe?
Red light and near infrared light therapy devices are promoted for numerous conditions, including acne, fibromyalgia, pain, cognitive function and other uses. Find out if these devices work and if they are safe.

Product Review
Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, Boswellia, Collagen and Turmeric)
18% of Joint Health Supplements Failed to Pass Our Tests. See Which Passed or Failed, and Our Top Picks.

CL Answer
Are light boxes effective for preventing and treating seasonal affective disorder (SAD), also known as "winter depression," or circadian rhythm disorders such as jet lag? Which light boxes are best?
Learn about the benefits and safety of light boxes (including who is most likely to benefit), how to use these devices, which commercially available products appear to meet the technical requirements, and who should not use light boxes.

Product Review
Fish Oil, Krill Oil, and Algal Oil Omega-3 (DHA & EPA) Supplements Review
See Our Omega-3 Top Picks and Avoid Rancid Fish Oils.

News Release
May 12, 2025
Best & Worst Manuka Honey Identified by ConsumerLab Tests
White Plains, NY, May 12, 2025 -- Manuka honey is often promoted for wound healing, and for soothing sore throats, cough, acid reflux, and other conditions, due to its high concentration of the antibacterial compound methylglyoxal (MGO), and other compounds derived from the manuka bush.
Product Review
Astaxanthin Supplements Review
See Which Astaxanthin Products Passed or Failed CL's Tests

Product Review
Black Currant, Borage, Evening Primrose, Flax and Hemp Seed Oil Review
Choose the Best Flaxseed and Other Oils Based on Our Tests.

Product Review
Collagen Supplements Review
See our Top Picks for Wrinkles and Joints

Product Review
Vitamin A Supplements Review, Including Beta-Carotene and Cod Liver Oil
Find the Best Vitamin A Supplement. Best Quality Vitamin A Supplements Identified, Including Cod Liver Oil.

Clinical Update
7/22/2025
Lactoferrin for Dry Eye?
Lactoferrin is a natural component of tears, but can taking lactoferrin as a supplement relieve symptoms of dry eye? Find out in our article about the health benefits of lactoferrin. Also learn about other supplements, eye drops, and lifestyle modifications for dry eye.
Clinical Update
6/02/2013
Glucosamine May Increase Eye Pressure
New research suggests that people with glaucoma or intraocular hypertension could have worsening eye pressure if they take a glucosamine supplement. Get the details (along with our tests of glucosamine and other joint health supplements) in the updated Glucosamine/Joint Health Supplements Review >>
CL Answer
L-Tyrosine: Health Effects and Safety
Tyrosine has been evaluated for conditions including physical and mental stress, phenylketonuria (PKU), depression, and attention deficit-hyperactivity disorder (ADHD). Find out if it works and if it's safe.

Product Review
Resveratrol Supplements Review (From Red Wine, Knotweed, and Other Sources)
See Which Resveratrol Supplements Were Best In Our Tests and Comparisons. Learn What Resveratrol Can and Can't Do.

Clinical Update
5/28/2019
Glucosamine & Eye Pressure
Glucosamine may increase eye pressure in people with glaucoma or intraocular hypertension. But does it have this effect in people who don't have these conditions? See what a new study found in the Concerns and Cautions section of the Joint Health Supplements Review. Also see our Top Picks for glucosamine and chondroitin.
Clinical Update
6/19/2025
Red Light Devices & Eye Protection
Find out when eye protection is recommended with red light therapy in the Concerns and cautions section of our article about red light therapy. Also learn about possible health benefits of red light therapy.
Clinical Update
1/25/2022
Goji Berries for the Eyes?
Goji berries are a good source of zeaxanthin, an anti-oxidant compound that, like lutein, may help protect the retina of eye. But does eating goji berries improve a measure of macular health (macular optical density) or improve vision? See what recent research showed in the Getting Lutein and Zeaxanthin from Food section of our Vision Supplements Review. Also see our Top Picks among vision supplements.
News Release
February 26, 2025
ConsumerLab Survey Shows 21 Most Popular Vitamins and Supplements
White Plains, New York, February 26, 2025 — A recent survey of more than 8,400 people who regularly use dietary supplements shows that among the 212 most popular supplements, those that experienced the greatest absolute growth in popularity during 2024 were biotin (+9.
CL Answer
Are L-carnosine supplements helpful and safe?
Learn about the potential health benefits of L-carnosine, as well as possible safety concerns, drug interactions and cost.

CL Answer
What is hesperidin, what is it used for and is it safe?
Learn about hesperidin, including whether its beneficial for heart health, problems with circulation, stroke, cognitive function, depression, cancer and other conditions, and find out if it is safe.

Product Review
Pomegranate Juice and Supplements Review Article
What Are the Benefits of Pomegranate Juice and Supplements? Find Out What Pomegranate Can and Cannot Do For Your Health.

CL Answer
Do any supplements reduce wrinkles, increase skin elasticity, or tighten the skin?
Learn about collagen, biotin, CoQ10, cocoa, soy isoflavones, phytoceramides, and other supplements for reducing wrinkles and improving aging skin.

CL Answer
What are the health benefits of chlorophyll and chlorophyllin, and are they safe?
Chlorophyll supplements are promoted for reducing body odor, preventing cancer, removing toxins from the body, improving digestive conditions and other uses, but do they work and are they safe? Find out and learn if such supplements actually contain chlorophyll.

CL Answer
Can castor oil eye drops improve or dissolve cataracts? Is castor oil or tea tree oil helpful for other eye conditions such as blepharitis or dry eye?
Can castor oil eye drops help cataracts or for other conditions such as blepharitis? Find out if castor oil eye drops are safe to use, and if they help for other conditions. ConsumerLab.com's answer explains.

CL Answer
What is EpiCor? Does it really "boost" the immune system and prevent colds?
EpiCor supplement info, including evidence from clinical studies on immune function and cold/flu prevention, safety, and price.

Product Review
GABA Supplements Review
Does GABA Work As a Supplement?

Product Review
Turmeric and Curcumin Supplements and Spices Review
See Our Top Picks Among Turmeric Products

CL Answer
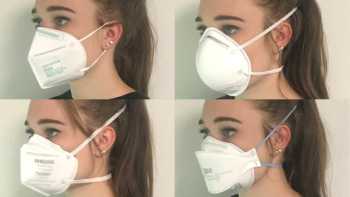
Which is the best mask to prevent COVID-19 and how do cloth, disposable, N95, and KN95 masks compare? How can I stop glasses from fogging?
See our Top Picks for masks. Learn how to make COVID-19 masks from materials at home that can be almost as effective as surgical mask and N-95 masks.
CL Answer
Is it safe to consume fish oil as a long-term food supplement?
Learn more about fish oil supplement safety including long-term use, side effects, and more.

Clinical Update
8/23/2022
Can-C Eye Drops for Dry Eye?
A product called Can-C Eye Drops has been promoted for improving dry eye as well as reducing the risk of cataracts. Does the evidence support this? Find in our updated CL Answer about supplements for dry eye.
Clinical Update
6/24/2025
Dry Eye: Do Warm Compresses Help?
Are warm compresses or microwaveable eye masks useful for relieving dry eye? Find out in our article about dry eye, which includes information about supplements, natural eye drops, and lifestyle modifications that may help.
Clinical Update
7/18/2025
Drops for Dry Eye
We’ve added information about common OTC eye drops to our article about supplements and eye drops for dry eye, including which ingredients are best for different types of dry eye and the pros and cons of preservatives.
CL Answer
Do any supplements help prevent or reduce eye floaters?
Find out eye floaters (vitreous floaters) be treated with supplements like vitamin C, lutein, ginkgo, milk thistle, collagen or hyaluronic acid, or with products such as VitreousHealth.
Clinical Update
7/28/2018
Dry Eye, Fish Oil and Contact Lenses
Taking fish oil can help people with dry eye and it was recently studied for dry eye associated with wearing contact lenses. Find out what the recent study found and learn more about fish oil for dry eye in the What It Does section of the Fish Oil & Omega-3 Supplements Review. (Also see our Top Picks among fish oil supplements.)
Clinical Update
7/08/2025
Is Castor Oil Safe for Eyes?
Some eye drops contain castor oil, but is it safe to apply regular castor oil to the eyes? Find out in our article about using castor oil for cataracts, dry eye, and blepharitis.
Clinical Update
11/28/2023
Enzymes for Eye Floaters
Does supplementing with digestive enzymes reduce eye floaters and improve sight? See what a recent study showed in the Eye Floaters section of our Digestive Enzymes Supplements Review.
Also see: Do any supplements help prevent or reduce eye floaters?
Clinical Update
5/21/2024
MSM Drops for Dry Eye?
Should you consider eye drops containing MSM for dry eye? Find out in our article about "natural" treatments and supplements for dry eye.
Clinical Update
8/19/2022
Supplements for Dry Eye
We added information about the following topics to our article about supplements and lifestyle modification for dry eye:
- Does coffee or caffeine supplements improve dry eye?
- Does krill or algal oil have similar effects as fish oil for dry eye disease?
Clinical Update
8/08/2023
Castor Oil Eye Drops
Are castor oil eye drops helpful for dry eye, blepharitis, or cataracts, and are they safe? Find out in our article about castor oil eye drops.
CL Answer
I was told I have a MTHFR gene mutation. Do I need to take methylated forms of B vitamins, such as methylfolate and methylcobalamin?
MTHFR gene mutation can impact B vitamin bioavailability. Learn more about this and which deficiencies are more likely to occur.

Recalls & Warnings
January 16, 2015
CVS Sued for Eye Health Supplement Claims
A class-action lawsuit has been filed against CVS for promoting the company's Advanced Eye Health supplement as comparable to the formula used in the large National Institutes of Health (NIH) eye disease study known as AREDS2, despite the fact that it does not contain the same ingredients.
Clinical Update
6/24/2015
Fish Oil for Dry Eye
A large study of computer-using young men and women experiencing dry eye found that fish oil significantly improved symptoms. Benefits have also been reported in postmenopausal women with dry eye. Details, including dosage, are found in the "What It Does" section of the Fish Oil Supplements Review >>
Clinical Update
9/21/2021
Enzyme Supplements for Eye Floaters?
Can enzyme supplements (bromelain, papain and ficin) reduce eye floaters? Find out what a recent study suggests in the What They Do section of our Digestive Enzyme Supplements Review.
Also see our answer to the question: Do any supplements help prevent or reduce eye floaters?
Clinical Update
9/07/2022
Supplement for Eye Floaters?
Did a supplement containing vitamin C, zinc, and other antioxidant ingredients help reduce eye floaters in a recent study? Find out in our article: Do any supplements help prevent or reduce eye floaters?
Clinical Update
9/16/2022
Gluten & Dry Eye?
A CL member commented that their chronic dry eye went away after eliminating gluten from their diet. Is there clinical evidence that this can help? Find out in our article about supplements and lifestyle modifications for dry eye.
Clinical Update
11/22/2022
Maqui Berry for Dry Eye?
Can supplementing with maqui berry extract (in products from LifeExtension, NOW, Swanson, and other brands) improve symptoms of dry eye? Find out what research suggests in our updated CL Answer about supplements for dry eye.
Clinical Update
8/16/2022
Water for Dry Eye?
Does drinking extra water help with dry eye? Find out in our updated article about dry eye -- which includes information about supplements and lifestyle habits than can help.
Clinical Update
3/11/2022
How Vitamin A Deficiency Can Affect the Eyes
Vitamin A deficiency was linked to eye floaters and loss of peripheral vision in a recently reported case. Get the details, and learn about other symptoms of vitamin A deficiency, in the What It Does section of our Vitamin A Supplements Review. Also see our Top Picks among vitamin A supplements.
See our answer to the question: Do any supplements help prevent or reduce eye floaters?
Clinical Update
6/14/2022
Fish Oil For Dry Eye?
Can taking fish oil reduce dry eye in older men and women? Find out what a recent study showed in the What It Does section of our Fish Oil Supplements Review.
Also see our CL Answer about Supplements for Dry Eye.
Clinical Update
5/17/2024
Can Omega-3 Help with Dry Eye?
Did taking an omega-3 fatty acid supplement for 3 months improve dry eye symptoms? Find out in the Dry Eye section of our Fish Oil/Omega-3 Supplements Review, which includes our Top Picks among omega-3 supplements.
Clinical Update
11/14/2023
Manuka Honey for Dry Eye?
Is manuka honey helpful for dry eyes when applied in an eye drop? Find out what research has shown in our article about manuka honey.
Also, find out if mixing manuka honey into hot beverages deactivates its key compounds.
Clinical Update
6/07/2024
Supplement for Dry Eye?
A new "dry eye supplement" decreased symptoms of dry eye in a recent study - but was it enough to stop using lubricating drops? Find out more in the What It Does section of our Vision Supplements Review, which includes our Top Picks among vision supplements.
Clinical Update
7/15/2025
Pineapple for Eye Floaters?
Does eating pineapple reduce eye floaters? Find out in the Eye Floaters section of our Digestive Enzyme Supplements Review.
Clinical Update
6/19/2025
Dry Eye: Do Blue Light Glasses Help?
Does wearing blue light glasses improve symptoms of dry eye when using digital devices? Find out what a recent study showed in our article about supplements, drops, and lifestyle modifications for dry eye.
Clinical Update
5/22/2025
Avoiding Eye Irritation from Sunscreens
Some sunscreens can cause eye irritation. Find out which types of sunscreen are least likely to cause eye irritation in our article about safe and effective sunscreens.
CL Answer
Are antioxidant supplements good or bad for you?
Learn more about whether antioxidants like astaxanthin, beta-carotene, coenzyme Q10, curcumin, resveratrol, selenium, vitamin A, vitamin C and vitamin E are safe and if they help prevent chronic diseases.

Clinical Update
11/30/2021
Benefits of Flaxseed Oil
Find out if flaxseed oil helps with eye floaters in our updated answer to the question: Do any supplements help prevent of reduce eye floaters?
CL Answer
What is Pycnogenol, does it work, and is it the same as other pine bark extracts?
Learn more about Pycnogenol, including evidence from clinical studies on blood clots, osteoarthritis, cognition, vision, tinnitus, and the skin and safety.

CL Answer
Does PrimeBiome Improve Aging Skin?
Is there evidence that PrimeBiome or its ingredients improve the appearance of skin, and is it worth its price? Find out.

Clinical Update
8/30/2022
Tea Tree Oil Toxicity
Is tea tree oil safe to use around the eye? Be careful -- it can potentially cause eye injury.
There is also concern that tea tree oil can cause growth of breast tissue in young boys. Learn more about this in the Cautions and Concerns section of our Lavender and Tea Tree Essential Oils Review.
Clinical Update
5/05/2025
Collagen for Eye Wrinkles?
Did taking a popular type of collagen reduce eye wrinkles in a recent study? Find out in our Collagen Supplements Review, which includes our Top Pick collagen supplements for skin and for joints.
Clinical Update
6/30/2025
Eyedrop Lawsuit Settlement
Consumers who purchased certain homeopathic eyedrops can submit a claim as part of a class-action lawsuit settlement. Get the details in our article about supplements, natural eye drops, and lifestyle modifications for dry eye.
Clinical Update
9/22/2020
Fish Oil and Eye Bleeding
Can high-dose fish oil increase the risk of bleeding in the eye? Get the details of a recently reported case in the Concerns and Cautions section of our Fish Oil Supplements review. Also see our Top Picks for fish oil.
Clinical Update
7/15/2017
Lutein May Reduce Eye Strain and Headaches
Taking a combination of lutein and other carotenoids may help reduce eye strain, fatigue, headaches and sleep problems associated with extended time spent in front of computer screens. Get the details in the "What It Does" section of our Vision Supplements Review. The Review includes our tests and quality comparisons of popular vision supplements.
Clinical Update
4/14/2018
Fish Oil for Dry Eye?
A major study evaluated the effects of supplementing with the omega-3 fatty acids EPA and DHA in people with dry eye. Get the results and details in the What It Does section of the Fish Oil Supplements Review. (Also see our Top Picks among fish oil supplements).
Clinical Update
7/13/2018
Fruit for the Eyes
Can consuming certain foods or beverages reduce the risk of developing age-related macular degeneration (AMD) — a common eye disease? Yes, according to a recent study. Get the details in the food section of the Vision Supplements Review. (Also see our Top Rated vision supplements.)
Clinical Update
11/01/2019
Eye Risk With High-Dose Niacin
Very high doses of niacin are sometimes used to lower high cholesterol, but there are risks -- including damage to the eye, as shown in a recent case. Get the details in the Niacin section of the B Vitamin Supplements Review.
Clinical Update
6/21/2015
Lutein and Visual Processing
Lutein and zeaxanthin are known to help certain people who suffer from macular degeneration, an eye disease. A new study suggests that they may also improve visual processing, perhaps through effects outside the eye. For more details of the study, including dosage, as well as our tests of supplements, see the Review of Vision Supplements with Lutein and Zeaxanthin Review >>
Clinical Update
8/04/2013
Supplement Helps Dry Eye
A supplement containing omega-6 (from black currant seed oil) and omega-3 fatty acids (from fish oil) was recently shown to improve symptoms of dry eye. Get the details about this formula (plus our test results of other omega-3 and -6 fatty acids supplements) in the updated Black Currant, Borage, Evening Primrose, and Flaxseed Oils Review >>
Clinical Update
12/14/2021
Fish Oil for Dry Eye?
Does supplementing with fish oil, alone or in combination with other oils, improve symptoms of dry eye? See the results of the latest study in the What It Does section of our Fish and Marine Oil Supplements Review. Also see our Top Picks among fish oil supplements.
CL Answer
What is the best sunscreen based on safety and efficacy?
We’ve boiled down the latest research about mineral and chemical sunscreens, including product tests, to help you choose the best sunscreen (which will depend on whether you expect to be in or out of the water). We also list popular sunscreens that have, or have not, been found to contain cancer-causing compounds (benzene and benzophenone).

CL Answer
Is ingesting colloidal silver helpful for any condition and is it safe to use?
Find out if there is evidence showing colloidal silver can help to treat infections, boost the immune system, help with arthritis and more, plus find out if colloidal silver can cause blue skin or other side effects.

Clinical Update
7/18/2024
Red Light Devices – More Answers
We've added more information to our recent article about red near infrared light devices about based on questions received:
- Vitiligo: Does red light help with vitiligo?
- Eye conditions: Is red light effective and safe for treating eye conditions , such as macular degeneration?
- Joint Pain: Is Move+ Pro by Kineon cleared by the FDA for reducing joint pain?
- Cleared by FDA? What is the difference between a device that has been "cleared" by the FDA as opposed to "approved" by the FDA?
- Product status: What is the FDA status of Omnilux Contour Face Mask, TenDlite, PainAway, and FibroLux?
- Devices vs. Sunshine? Since sunshine includes the wavelengths from red and near infrared light devices, why not just get sun exposure?
CL Answer
Does ergothioneine help prevent age-related problems such as impaired cognition, Alzheimer’s disease, Parkinson’s disease or cataract, and is it safe?
Learn about ergothioneine and its potential use for cognitive impairment, dementia, Alzheimer's disease, Parkinson's disease, cataract, or kidney disease, as well as its safety and cost of supplements containing this ingredient.

Recalls & Warnings
July 23, 2025
FDA Warns Seller of Allegro Eye Drops, Tea Tree Oil Eyelid Wipes, and More
On July 9, 2025, the FDA issued a Warning Letter to Scope Health Inc.
CL Answer
What are the health benefits of coconut oil and medium chain triglycerides, such as those used in Bulletproof Coffee?
Learn the health benefits of coconut oil and medium chain triglycerides (MCTs), including evidence from Bulletproof Coffee, clinical studies on obesity, skin, and Alzheimer's disease.

Clinical Update
1/28/2022
Symptoms of Vitamin A Deficiency
Although not common, be aware of signs of vitamin A deficiency involving the eyes, skin, and nails, as exemplified by a recent case. Get the details (including medical conditions which can cause it) in the What It Does section of our Vitamin A Supplements Review. Also see our Top Picks among vitamin A supplements.
Clinical Update
8/29/2023
Counterfeit Vision Supplement
Counterfeit versions of a major brand of eye vitamin have been sold on Amazon. For details, see the Update in our Vision Supplements Review, which includes our Top Picks among vision supplements.
Also see: How to Avoid Buying Counterfeit Vitamins and Supplements
Clinical Update
7/19/2022
Quercetin for Seasonal Allergies?
Did quercetin improve nasal and eye symptoms among people with seasonal allergies in recent study? Find out in the Seasonal Allergy section of our Quercetin Supplements Review.
Clinical Update
12/20/2022
Collagen for Wrinkles?
How much did collagen supplementation reduce the severity of "crow’s feet" wrinkles around the eyes in a recent study? Find out in the Aging skin and wrinkles section of our Collagen Supplements Review. Also see our Top Picks among collagen supplements for skin and for joints.
Clinical Update
3/31/2025
Cocoa for Aging Eyes?
Did taking cocoa extract slow macular degeneration (AMD) in older people? Find out what a recent study showed in the Vision section of our Dark Chocolates, Cocoa & Cacao Powders, Nibs, and Supplements Review, which includes our Top Picks among supplements, cocoas and mixes, dark chocolates and chips, and cacao nibs.
Also see our Top Picks AREDS formulas to slow the progression of AMD in our Vision Supplements Review.
Clinical Update
9/19/2024
Propolis for Vision?
Propolis is found in some eye formulas, but is there good evidence that is helps with conditions such as glaucoma, cataracts, or retinal damage? Find out in our Vision Supplements Review, which includes our Top Picks among lutein and AREDS formula supplements.
Also find out if propolis has shown benefit for fatty liver disease or arthritis.
Clinical Update
10/21/2024
Pistachio Eye Benefits?
Did eating pistachios, a natural source of lutein, improve macular pigment density in the retinas of people with low density levels (a risk factor for age-related macular degeneration)? Find out what a recent study showed in the Macular Degeneration section of our Vision Supplements Review, which includes our Top Pick lutein and AREDS2 supplements
Also see: Do pistachios provide melatonin?
Clinical Update
4/23/2024
Hair Devices & Safety
Can low-level light therapy (LLLT) hair devices such as Theradome or Capillus cause eye damage? Find out in our article about hair supplements and devices.
Clinical Update
2/06/2024
Omega-7s for Wrinkles?
Did taking an omega-7 supplement decrease wrinkles around the eyes (“crow’s feet”) in a recent study? Find out in our Fish, Krill, and Algal Oil Supplements Review, which includes our Top Pick for getting omega-7s.
Also see: Do any supplements increase skin elasticity or tighten the skin?
Clinical Update
1/19/2024
Pycnogenol for Hearts and Eyes?
We answered the following questions in our article about Pycnogenol and pine bark extracts:
- Does Pycnogenol help control coronary artery calcification?
- Is Pycnogenol beneficial for glaucoma?
Clinical Update
10/31/2020
Fish Oil & Vitamin D for Macular Degeneration?
Can taking fish oil and vitamin D reduce the risk of age-related macular degeneration (AMD)? See what a large study found in the Eye Disease section of the Fish Oil Supplements Review. Also see the Vision Supplements Review for our tests of vision supplements, including those that most closely resemble the AREDS2 formula and our Top Picks among products.
Clinical Update
1/19/2014
Multivitamin Lowers Cataract Risk
A large, long-term study using a low-dose multivitamin found that it reduced the risk of developing cataract (a clouding of the lens of the eye) by 9% compared to placebo. Interestingly, the reduced risk of cataract was negated when a separate vitamin C supplement was also taken. For details about the study and dosage, as well as our tests and comparison of multivitamins, see the updated Multivitamin and Multimineral Supplements Review.
Clinical Update
4/20/2014
Probiotic Helps a Little with Allergy
Among people already taking a daily antihistamine, adding a daily probiotic provided a small, additional benefit -- particularly with ocular (eye) symptoms, according to a new study. For more information about the probiotic used, and our tests of probiotic supplements, see the updated Probiotic Supplements Review >>
Clinical Update
11/08/2015
When to Use Lutein
Lutein and zeaxanthin can help people who have macular degeneration of the eye. However, a recent study found that taking lutein and zeaxanthin may only help those with existing low levels of these compounds. Get more details, including dosage, in the Lutein and Zeaxanthin Review >>
Clinical Update
8/23/2016
Omega-3s and Diabetic Eye Disease
Getting the omega-3 fatty acids EPA and DHA from eating fish is associated with a much lower risk of developing sight-threatening retinopathy in diabetics, according to a recently published study. The same benefit has been shown in non-diabetics. But do omega-3 supplements help? Find out in the "What It Does" section of the Fish Oil Supplements Review >>
Clinical Update
4/10/2011
Fish Oil and the Eye
A study of over 30,000 women showed that the risk of developing age-related macular degeneration (AMD) was reduced by up to 42% among those consuming the most fish and highest amounts of omega-3 fatty acids DHA and EPA. Find out the specific types and amounts of fish and the amounts of DHA and EPA associated with the greatest reductions in the risk of AMD. See the Fish Oil Supplements Review for more >>
Clinical Update
8/03/2019
Concerns With Colloidal Silver
Consuming colloidal silver may affect the eyes and cause a type of vasculitis, according to recently published reports. Get the details in our answer to the question: Is ingesting colloidal silver helpful for any condition and is it safe to use?
Clinical Update
7/03/2020
Lycopene for Wrinkles?
A recent study tested the effects of lycopene supplementation on wrinkles around the eyes ("crow's feet") in older women. Find out if it helped in the What It Does section of the Lycopene Supplements Review. Also see our Top Pick among lycopene supplements.
Clinical Update
3/07/2020
Melatonin and Cataracts
Do you have cataracts (clouded lenses of the eye)? A recent study suggests that having them removed and replaced with a certain type of intraocular lens may increase natural melatonin production. For details, see the ConsumerTips section of the new Melatonin Supplements Review. Also see our Top Picks among melatonin supplements.
Clinical Update
4/28/2020
Early Sign of Thiamin Deficiency
Thiamin (vitamin B1) deficiency can cause lasting neurologic damage and affect heart function. It can occur with poor nutrition and as a result of taking certain diuretics. A recent case highlighted an early sign of thiamin deficiency that involves the eyes. We've added this information to the Thiamin section of the B Vitamin Supplements Review. Also see our Top Pick for thiamin and B complexes that contain thiamin. You can also get thiamin from a good multivitamin.
Clinical Update
9/03/2019
Collagen for Wrinkles?
A new study put a popular branded collagen ingredient to the test, measuring its effects on collagen content in the skin and on facial wrinkles, including wrinkles around the eyes. See the results in our updated answer to the question: Do collagen or hyaluronic acid supplements really help aging or wrinkled skin?
Clinical Update
10/01/2017
Does Lutein Help?
Lutein is an antioxidant that helps protect the retina of the eye and can slow the progression of macular degeneration. But does taking lutein help if you already have normal macular density? Two studies have "looked" at this question (including one published this week) and, you can "see" the answer in the "What It Does" section of Vision Supplements (Lutein & Zeaxanthin) Review >>
Clinical Update
2/25/2017
Lutein for Stress?
Supplementing with lutein can help some people protect against macular degeneration of the eye. A new study suggests it may also reduce stress. For details, see the "What It Does" section of the Review of Vision Supplements with Lutein and Zeaxanthin (which includes our tests of products) >>
Clinical Update
10/02/2021
Sanitizer Warning
Consumers are being warned not to use a specific hand-held UV sanitizing wand that could cause skin or eye injury within seconds of use. Details are in our updated article about UV Light Sanitizers and COVID.
CL Answer
Is it better to take fish oil, flaxseed oil -- or both?
Fish oil and flaxseed oil -- how they are different, what they are used for, if and how they can help with arthritis, depression, lowering cholesterol & more.

Recalls & Warnings
September 08, 2025
FDA Warns Two Sellers of Homeopathic Eye Drops
On August 25, 2025, the FDA issued Warning Letters to Supply Center USA and Homeopathic Educational Services (dba Homeopathic Family Medicine) following reviews of the companies’ websites, which found statements about certain "homeopathic" eye drop products to be drug claims.
CL Answer
What are the health benefits of palmitoylethanolamide (PEA), and is it safe?
Palmitoylethanolamide (PEA) is commonly promoted for its anti-inflammatory and pain-relieving effects. Find out if it works.

Recalls & Warnings
November 29, 2023
Dr. Berne’s Whole Health Products Warned for Contaminated Eye Drop Products, Drug Claims
On November 22, 2023, the FDA issued a Warning Letter to Dr. Berne’s Whole Health Products following review of the company’s website, which found statements about the company’s Dr. Berne’s MSM Eye Drops 5%, Dr. Berne’s MSM Eye Drops 15%, and Dr.
Recalls & Warnings
November 09, 2023
Zazzee Naturals Warned for MSM Eye Drop Claims
On October 30, 2023, the FDA issued a Warning Letter to Dexterity Health, LLC DBA Zazzee Naturals following review of the company’s Amazon storefront, which found statements about the company’s Liquid MSM Drops to be drug claims.
Recalls & Warnings
February 28, 2024
Microbial Contamination Found in LightEyez MSM Eye Drops
On February 15, 2024, the FDA issued a Warning Letter to LightEyez Limited after testing by the agency found the company’s LightEyez.com MSM Eye Drops V2.0 — Eye Repair (MSM Eye Repair Drops) to have microbial contamination in samples of the drops.
CL Answer
Himalayan and Sea Salts: Health Benefits, Toxic Metals, & Microplastics
Himalayan salt health benefits, along with other gourmet salts like Hawaiian and Mediterranean. Info including what's in them and their safety.

News Release
February 25, 2025
Top-rated Vitamin and Supplement Brands and Merchants for 2025 Based on Consumer Satisfaction
White Plains, New York, February 25, 2025 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 8,400 responses collected in November/December 2024.
CL Answer
What are phytoceramides? Do phytoceramide supplements really work to improve aging skin?
Phytoceramide supplement information, including what they are, what they claim to do, and if they have anti-aging effects.

CL Answer
Can fisetin, also called Cognisetin and Novusetin, really improve memory?
Fisetin (Cognisetin and Novusetin) information, including evidence from clinical studies on memory improvement, brain health, dosage, and pricing.

CL Answer
Which supplements are best for seasonal allergies?
Find out which supplements are best for allergies and can help reduce seasonal allergy symptoms, including bromelain, curcumin, vitamin D, and CLA.

CL Answer
What are the health benefits of green tea, and is it safe?
ConsumerLab explains green tea's potential health benefits for everything from fighting cancer to helping your heart.

Recalls & Warnings
December 07, 2023
Belmont Eyecare Warned for Colloidal Silver, MSM, and Castor Oil Eye Drop Claims
On December 1, 2023, the FDA issued a Warning Letter to Belmont Eyecare LLC, following a review of the company’s website, which found statements about the company’s Colloidal Silver Eye Drops, MSM Eye Drops, Organic Daytime Oil Eye Drops, Organic Daytime Oil Eye Drops Small, Organic ...
News Release
January 23, 2025
ConsumerLab Tests Identify Best Black Currant, Borage, Evening Primrose Flax and Hemp Oils
White Plains, NY, January 23, 2025 — Black currant, borage, evening primrose, flax, and hemp oils contain omega-3, -6, and/or -9 fatty acids that, when replacing saturated fats in the diet, may help reduce cardiovascular risk.
CL Answer
Estrogen Creams for Wrinkles?
Find out if estrogen creams can help reduce wrinkles, whether they are safe, and how "prescription" estriol and estradiol creams and gels differ from those sold over-the-counter.

CL Answer
What is lumbrokinase, does it have heart benefits, is it safe, and what should you look for, or avoid, in a lumbrokinase supplement?
Lumbrokinase is marketed as promoting healthy circulation and supporting heart health. Find out how and if lumbrokinase supplements actually work, possible safety concerns, and what to consider when choosing a lumbrokinase supplement.

Recalls & Warnings
September 14, 2023
FDA Warns CVS, Walgreens, Similasan & Others for Eye Drop Violations
On September 11, 2023, the FDA issued Warning Letters to the following eight sellers of homeopathic and other types of eye drops regarding the products noted in italics due to a variety of violations of FDA regulations, most notably that they were marked with claims suggesting that they could cure, ...
Recalls & Warnings
August 28, 2023
Dr. Berne’s MSM and Castor Oil Eye Drops Recalled Due to Bacterial and Fungal Contamination
On August 26, 2023, Dr. Berne’s Whole Health Products issued a recall of all lots of its MSM DROPS 5%, 15% Solution, Dr. Berne’s Organic Castor Oil Eye Drops and Dr. Berne’s MSM MIST 15% Solution due to the risk of bacterial and fungal contamination.
News Release
August 12, 2024
Too Much Vitamin D? Some Supplements Contain Much More Than Claimed, According to ConsumerLab
White Plains, NY, August 12, 2024 — Getting adequate vitamin D is important to maintaining bone and cardiovascular health, and may have other benefits. However, getting too much vitamin D can have adverse effects, including increasing the risk of fractures and falls.
CL Answer
Are vitamins and supplements from Canada of better quality because they are licensed?
Find out if Canadian vitamins and supplements are better quality because they are licensed, what it means to for a product to have an Natural Product Number (NPN) in Canada, and if vitamins and supplements in Canada are tested for quality and purity. ConsumerLab.com's answer explains.

News Release
July 31, 2024
Best Calcium & Bone Health Supplements According to ConsumerLab Tests
White Plains, NY, July 31, 2024 — Calcium is essential to maintaining bone health and plays critical roles in nerve transmission, muscle contraction, and the cardiovascular system.
News Release
July 30, 2024
ConsumerLab Tests Reveal Best Vitamin K Supplements
White Plains, NY, July 30, 2024 — Vitamin K helps with proper blood clotting as well as with calcium utilization in bones and the cardiovascular system.
News Release
April 11, 2024
Best CoQ10 & Ubiquinol Supplements According to ConsumerLab Tests
White Plains, NY, April 11, 2024 — CoQ10 and its active form, ubiquinol, are among the most popular supplements, taken by 42.2% of supplement users, often taken by people on cholesterol-lowering statin medication and for a variety of other conditions.
News Release
February 29, 2024
Lead Found In Psyllium Fiber Supplements
White Plains, NY, February 29, 2024 — Psyllium is an effective laxative that may also have heart health benefits, but ConsumerLab’s recent tests found lead contamination in every psyllium fiber supplement it purchased and tested.
News Release
February 26, 2024
Latest ConsumerLab Survey Shows Growth in Popularity of Magnesium and Several Smaller Supplements
White Plains, New York, February 26, 2024 — A recent survey of more than 10,000 people who regularly use dietary supplements shows that supplements that experienced the greatest absolute growth in popularity during 2023 were pregnenolone (+7.5 percentage points), magnesium (+4.8 pts), berberine (+4.
News Release
February 25, 2024
Top-rated Vitamin and Supplement Brands and Merchants for 2024 Based on Consumer Satisfaction
White Plains, New York, February 25, 2024 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on over 10,000 responses collected in November/December 2023.
News Release
February 15, 2024
Unveiling the Truth About Green Tea’s EGCG Content
White Plains, NY, February 15, 2024 — ConsumerLab.com, a trusted name in health and wellness, sheds light on the EGCG (epigallocatechin gallate) content in green tea.
CL Answer
Do any supplements or diets help prevent or treat osteoporosis?
If you have osteoporosis, you may be interested in supplements or diets for bone health. Find out which can increase bone strength and density.

CL Answer
What are the benefits of grape seed extract?
Learn about the health benefits of grape seed extract, and find out it improves venous insufficiency, leg swelling, swelling after surgery, or can help to lower blood pressure.

News Release
December 28, 2023
10 Most Popular ConsumerLab Product Reviews of 2023
White Plains, New York, December 28, 2023 — In 2023, ConsumerLab tested more than two hundred products, adding to its extensive Product Reviews covering 150 categories of supplements, foods, and other health products. Among these, the following 10 were the most popular in 2023:
CL Answer
I'd like to know which supplements may help with my different medical conditions. What's the best way to look this up on ConsumerLab.com?
Learn more about how to use ConsumerLab.com to get the best information regarding supplements and brand testing. Our answer explains.

CL Answer
Do sea moss and carrageenan have health benefits? Are there risks?
Sea moss is promoted for thyroid health, boosting immunity, improving gut health, and other uses. Find out if it works and learn about possible side effects of sea moss and its constituent, carrageenan.

CL Answer
What are the health benefits of dark chocolate?
Information on the health benefits of dark chocolate, including how dark chocolate may improve heart health, decrease blood sugar levels and more. ConsumerLab.com's answer explains.

CL Answer
What are the benefits of Daily Synbiotic by Seed Health? Does it do what it claims, and is it better than other probiotics and prebiotics?
Review of Daily Synbiotic by Seed, including information about the prebiotic and probiotic strains in the supplement, and whether there is evidence of benefit for the synbiotic.

CL Answer
What is BPC-157, what is it used for and is it safe?
Find out about BPC-157, including what it's promoted for and if it is safe.
CL Answer
Does Arthrozene help with joint pain, and is it safe?
Arthrozene by Fisico is promoted for joint stiffness, soreness and discomfort. Find out if it is likely to help and if it is worth the cost.

News Release
November 02, 2023
Spirulina and “Greens” Supplement Tests Show Some Problems
White Plains, New York, November 2, 2023 — ConsumerLab tests of spirulina, chlorella, and “greens” supplements revealed that tablets of three brands of spirulina failed to disintegrate within the allotted time, and several other products contained amounts of lead unsuitable for regular consumption ...
CL Answer
What are the health benefits of luteolin, and is it safe?
Find out if luteolin, a compound found in many fruits and vegetables, is beneficial for cancer, heart health, skin aging, age-related cognitive decline, and other conditions, and learn if it's safe.

CL Answer
What are the best bug sprays to prevent tick and mosquito bites? Is the insecticide permethrin safe to use?
Find out how permethrin sprays differ and which can be used on sheets and sleeping bags versus clothing

CL Answer
Do any supplements or lifestyle changes reduce the symptoms of tinnitus? Is it true that some supplements can cause tinnitus?
Learn which supplements can ease tinnitus, including melatonin and pine bark extract. Understand which may actually cause tinnitus.

CL Answer
I live in Canada. Does ConsumerLab.com ever test Canadian products?
Find out which Canadian products ConsumerLab.com has tested, including Jamieson, Natural Factors, Webber Naturals and Lacteeze. Our answer explains.

CL Answer
Which supplements help with arthritis?
Learn more about supplements for joint health, including products such as Wobenzym and Zyflamend and ingredients such as collagen, curcumin, devil's claw, glucosamine and chondroitin, hyaluronic acid, magnesium, omega-3 fatty acids, palmitoylethanolamide (PEA), SAMe, and others.

CL Answer
Bee pollen: Purported health benefits and possible side effects
Bee pollen is promoted for numerous health conditions, including supporting immune health and boosting energy. Find out if it works and if it's safe.

CL Answer
Does Rooibos Tea Have Health Benefits and Is It Safe?
Learn about the health benefits of rooibos tea, including red and green, unfermented rooibos tea, how is differs from black and green tea, safety, and potential drug interactions with rooibos tea.

News Release
October 24, 2023
Best Fish Oil and Omega-3 Supplements? ConsumerLab Tests Reveal Some Are Rancid
White Plains, New York, October 24, 2023 — Recent ConsumerLab tests of popular fish, krill, and algal oil supplements revealed several products to be rancid. Fish and marine oils that are rancid, or “oxidized,” can have an extremely unpleasant odor and taste, and may be less safe and effective.
News Release
July 13, 2023
Best Vision Supplements According to ConsumerLab Tests
White Plains, New York, July 13, 2023 — Studies show that supplements containing lutein, zeaxanthin, zinc, and other ingredients can help slow the progression of age-related macular degeneration, which is the leading cause of blindness in adults over age 60.
News Release
May 25, 2023
Best and Worst Multivitamins According to ConsumerLab – Nearly 30% Fail Testing
White Plains, New York, May 25, 2023 — Recent ConsumerLab tests revealed problems with nearly 30% of the popular multivitamin and multimineral supplements selected for testing. Gummy vitamins were particularly likely to have issues.
CL Answer
Do any supplements help relieve stress?
Are there actually supplements for stress relief and panic attacks? Learn more about holy basil, fish oil, L-theanine, and ginseng.

News Release
April 10, 2023
Best and Worst Olive Oils According to ConsumerLab Tests
White Plains, New York, April 10, 2023 — Extra virgin olive oil can be a heart-healthy alternative to saturated fats in the diet, providing antioxidant polyphenols and plenty of oleic acid, a “healthy” monounsaturated fat.
News Release
February 25, 2023
Top-rated Vitamin and Supplement Brands and Merchants for 2023 Based on Consumer Satisfaction
White Plains, New York, February 25, 2023 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,600 responses collected in November/December 2022.
CL Answer
My dog is getting older and his veterinarian recently recommended giving him a glucosamine supplement for his joints. Has ConsumerLab.com tested these, or other supplements for pets?
Find out our test results on glucosamine for dogs and cats as well as other supplements for pets.

CL Answer
Sugar Substitutes: Pros, Cons, and Best Choices
Learn about the pros and cons of using sugar substitutes and artificial sweeteners in place of table sugar. Sweeteners discussed include stevia, monk fruit, and other high-intensity sweeteners; xylitol, erythritol, allulose and other low-calorie sweeteners; and agave syrup, black sugar, coconut sugar, honey, molasses and other sugar alternatives.

CL Answer
What are the health effects of apigenin and is it safe?
Apigenin, a flavonoid compound, is promoted for sleep, depression, cancer, and other conditions. Find out if it works and if it's safe.

CL Answer
Schisandra Berry Supplements: Health Benefits and Safety
Schisandra berry extracts and supplements are promoted to improve mood and sleep, liver health, muscle strength, and symptoms of menopause. Find out if they work and are safe, including side effects and drug interactions.

CL Answer
Are Hormone Harmony and other Happy Mammoth Supplements Worth Taking?
Are the supplements Hormone Harmony, Hormone Harmony Plus+, Prebiotic Collagen Protein, or Bloat Banisher by Happy Mammoth likely to be beneficial for menopause symptoms or digestive health? Find out.

CL Answer
Which deodorants and antiperspirants are contaminated with benzene and which are not?
Benzene, a carcinogen, has been detected in over 40 spray deodorants, antiperspirants, and body sprays. Tests were conducted on brands including Axe, Bath & Body Works, Brut, Degree, Dove, Gold Bond, Right Guard, Secret, Speed Stick, Suave, Old Spice, Victoria's Secret and others. Find out which products contained benzene, and how much they contained.
CL Answer
Can any supplements be used to reduce inflammation in people sensitive to NSAIDs such as ibuprofen, high-dose aspirin, or naproxen?
Find out if supplements such as turmeric, fish oil, MSM (methylsulfonylmethane), SAMe (S-adenosyl-methionine), boswellia, ginger, white willow bark or arnica may, or may not, be helpful to people sensitive to NSAIDs.

CL Answer
Does OsteoMD improve calcium absorption and increase bone density?
ConsumerLab reviews the evidence for OsteoMD by 1MD, a bone health supplement promoted to slow bone loss and reduce the risk of osteoporosis.

CL Answer
Is hyaluronic acid helpful for osteoarthritis?
Hyaluronic acid supplement information including joint pain, osteoarthritis, clinical evidence, dosage and more.

CL Answer
The suggested daily serving for my fish oil supplement, three 1,000 mg softgels, seems like a lot. Do I really need to take this much?
Information on the proper fish oil dosage per day. See the best dosage for different uses, such as lowering triglyceride levels or improving mood, plus information on overdose.

Recalls & Warnings
September 26, 2012
Maker of Eye Health Supplements Warned for Drug Claims
On September 18, 2012, the FDA issued a warning letter to EyeScience Labs, L.L.C. because statements made about the company's Macular Health Formula, Dry Eye Formula and Diabetic Vision Formula supplements were found to constitute drug claims.
Recalls & Warnings
February 06, 2023
Recalled Eye Drops Linked to Vision Loss and One Death
On February 2, 2023, Global Pharma Healthcare issued a nationwide recall of its Artificial Tears Lubricant Eye Drops due to potential contamination with bacteria that can cause serious infection, blindness, and death.
Recalls & Warnings
July 19, 2024
Walnuts Recall Due to Listeria
On July 19, 2024, Stutz Packing Company recalled its 1-pound packages of Shelled Walnuts due to potential contamination of Listeria monocytogenes. Routine sampling realized that the finished products contained bacteria. Currently, there have been no reports of illness related to this call.
News Release
February 24, 2023
Probiotics Rise in Popularity as Vitamin C, Melatonin, and Others Dip in Latest ConsumerLab Survey of Supplement Users
White Plains, New York, February 25, 2023 —A recent survey of 8,600 people who regularly use dietary supplements shows that probiotics (+3.04 percentage points), quercetin (+2.3 pts), and vitamin K (+1.
Recalls & Warnings
July 22, 2022
FDA Warns Seller of Vision, Calcium & Magnesium Supplements and More
On June 14, 2022, the FDA issued a warning letter to New Sun Inc. following inspection of the company’s website which found statements about the company’s products to be drug claims.
Recalls & Warnings
August 28, 2024
Now Foods Recalls Raw Brazil Nuts Due to Mold and Yeast
On August 14, 2024, NOW Foods issued a recall of its Now Foods Whole & Unsalted Raw Brazil Nuts because they were found to be contaminated with mold and yeast.
Recalls & Warnings
May 01, 2024
Organic Walnuts Recalled Due to E. Coli Risk
On April 30, 2024, Gibson Farms recalled two lots of Organic Light Halves and Pieces shelled walnuts due to potential contamination with E. coli (Escherichia coli).
Recalls & Warnings
April 03, 2024
FDA Warns Ambaya Gold for Promoting Products for Depression, Cancer, & Arthritis
On December 5, 2023, the FDA issued a Warning Letter to Ambaya Gold Health Products, LLC following review of the company’s website and social media, which found statements about the company’s Brain Balance, Immune System Boost, Dentist In A Bottle, Essensiac, Fulvic Green, Silver ...
News Release
February 23, 2023
Best GABA Supplements Identified by ConsumerLab
White Plains, New York, February 23, 2023 — GABA (gamma-aminobutyric acid) supplements are claimed to have a "calming" effect, and are often promoted to reduce stress and insomnia and improve cognitive performance.
News Release
December 16, 2022
Is Your Chocolate or Cocoa Healthful or Toxic? ConsumerLab Finds Major Differences Among Products.
White Plains, New York, December 16, 2022 — Although dark chocolate and cocoa can be good sources of potentially heart-healthy, antioxidant flavanols, they can also be contaminated with toxic heavy metals such as cadmium and lead – and consumers can’t judge products from their labels.
Recalls & Warnings
August 25, 2022
Oregon’s Wild Harvest Warned by FDA for Manufacturing Violations
On July 8, 2022, the FDA issued a warning letter to Oregon’s Wild Harvest, Inc.
Recalls & Warnings
June 12, 2023
Children's Liquid Vitamin Supplement Recalled
On June 7, 2023, Procaps S.A. de C.V. issued a voluntary recall of various lots of Laboratorios Lopez’s Bacaolinita 8 fl. oz children's liquid vitamin supplement because it contains undeclared PEG-40 hydrogenated castor oil.
Clinical Update
11/11/2022
Health Benefits of a Natural Sweetener?
Does lucuma powder, a natural sweetener, have particular health benefits? Find out in the Other Natural Sweeteners section of our answer to the question: What are the health benefits of stevia?
Clinical Update
11/03/2023
Collagen For Bone Health?
Is there evidence that collagen peptides (as in Fortibone) strengthen bones? Find out in the bone health section of our Collagen Supplements Review. Also see our Top Picks for collagen for skin and joint health.
Also see: Do any supplements or diets help prevent or treat osteoporosis?
Clinical Update
3/08/2024
Newly Approved Bone Health Supplement
We recently tested NuBest Tall 10+, a bone health supplement that contains calcium, vitamin D, and vitamin K. It was Approved for Quality and is now in our Calcium and Bone Health Supplements Review.
The product was tested and Approved through ConsumerLab's Quality Certification Program.
Recalls & Warnings
May 08, 2024
APG SEVEN Warned for Promoting Products for Cancer, Depression, Arthritis, & More
On April 18, 2024, the FDA issued a Warning Letter to APG SEVEN, INC following a review of the company’s product labels, website, and social media, which found statements about the company’s BioMoringa, BioDiabetin, Maca Plus, BioProstate, Immune Support, HongoTrap, Honbacterol, ...
News Release
October 06, 2022
Lion’s Mane and Chaga Supplement Labels May Mislead, According to ConsumerLab Tests
White Plains, New York, October 6, 2022 — Lion’s mane and chaga supplements are often promoted to improve memory and mood, boost the immune system, and a range of other health benefits.
News Release
May 16, 2022
Best Calcium and Bone Health Supplements Revealed by ConsumerLab Tests
White Plains, New York, May 16, 2022 — Calcium is essential for maintaining bones and plays critical roles in nerve transmission, muscle contraction, and the cardiovascular system.
News Release
April 13, 2022
ConsumerLab Tests Vitamin D Supplements and Cautions About Dose
White Plains, New York, May 12, 2022 — Getting sufficient vitamin D is essential for healthy bones and has a wide range of other health benefits. It's no wonder that it has been the #1 supplement in popularity year-after-year in ConsumerLab's survey of supplement users.
News Release
April 02, 2022
ConsumerLab Discovers Less Vitamin K Than Listed in Some Supplements
White Plains, New York, May 16, 2022 — Vitamin K plays an important role in proper blood clotting and calcium utilization in bones and is a common ingredient in bone health supplements.
Clinical Update
8/15/2023
Coffee & Bone Health
Does drinking coffee decrease bone density or worsen symptoms of arthritis? Find out in our article about coffee and bone health.
Clinical Update
1/31/2023
Supplements for Heart Health
We added information about whether certain supplements lower the risk of calcification in the coronary artery to our CL Answer about supplements for heart health.
Recalls & Warnings
June 30, 2022
FDA Warns Seller of Vision and Allergy Supplements
On May 26, 2022, the FDA issued a warning letter to Golden Lab LLC following an inspection of the website, which found statements about the company’s DoctoRx’s Optimal Formula Ocular Pressure & Optic Nerve Support Formula Ocular Health Capsule, DoctoRx’s Optimal Formula ...
Clinical Update
11/13/2011
Resveratrol Improves Health Measures
A recently published study of obese men given resveratrol (sometimes referred to as "red wine extract") showed improvement in certain parameters of health. The study used a lower dose than often suggested. For details, see the What It Does and What to Consider When Buying and Using sections of the Resveratrol Supplements Review.
Clinical Update
6/13/2014
Contaminated Chondroitin
A chondroitin ingredient on the market has been found to be contaminated with an inexpensive industrial compound. Get the details, along with our tests and comparisons of glucosamine and chondroitin supplements for joint health, in the update to the Review of Glucosamine, Chondroitin, MSM and Boswellia Supplements for Joint Health>>
Clinical Update
7/21/2018
Fish Oil for Heart Health?
Are fish oil supplements good for the heart? See what a review of over 70 clinical trials concluded in the Heart Attack and Stroke section of the Fish Oil and Omega-3 Supplements Review.
Also, learn what fish oil can or cannot do for other health conditions, such as arthritis, in the What It Does section of the review. (See our Top Picks among fish and marine oils.)
Clinical Update
3/09/2019
Boswellia for Osteoarthritis
Boswellia extract may reduce the pain and stiffness of knee osteoarthritis. See results of a recent study in the What It Does section of the Joint Health Supplements Review. Also see our Top Picks for Boswellia and other joint health supplements.
Clinical Update
5/15/2019
Glucosamine and Heart Disease
Does taking glucosamine affect your risk of developing cardiovascular disease? See what a large study recently found in the What It Does section of the Joint Health Supplements Review (Glucosamine, Chondroitin, MSM and Boswellia). Also see our Top Picks among joint health supplements.
Clinical Update
8/30/2019
Glucosamine for Hand Osteoarthritis?
A new study suggests that glucosamine may help people with hand osteoarthritis. Get the details in the What It Does section of the Joint Health Supplements Review. Also see our Top Picks for joint health supplements.
Clinical Update
3/03/2020
Do Glucosamine & Chondroitin Work?
After reviewing the clinical literature regarding glucosamine and chondroitin for osteoarthritis, an expert group drew conclusions regarding their efficacy. Find out what they concluded in the What It Does section of the Joint Health Supplements Review. Also see our Top Picks for glucosamine, chondroitin and other joint health supplements.
Clinical Update
12/03/2021
Pesticides in Coffee?
A member recently asked if coffee beans or ground coffee might contain enough pesticide residue to be a health concern. Find out in our updated CL Answer about Coffee and Heart Health.
Clinical Update
10/29/2021
Booster Recommended for People With Certain Mental Health Conditions
A booster is now recommended to people with certain mental health conditions. Learn what research prompted this change.
Clinical Update
2/14/2023
Hawthorn for Heart Health?
Can hawthorn berry supplements lower cholesterol and improve heart health? Find out in our updated CL Answer about cholesterol lowering supplements.
Also find out if hawthorn berry lowers blood pressure in our article about supplements for lowering blood pressure.
Clinical Update
2/07/2023
Strontium and Blood Tests
Strontium is included in some "bone health" supplements, but be aware that very high doses of strontium may interfere with blood tests for calcium and bone density scans. Get the details in the Concerns and Cautions section of our Calcium and Bone Health Supplements Review, which includes our Top Picks among products.
Also see: Which vitamins and supplements should be stopped before getting blood work and laboratory tests?
Clinical Update
5/09/2023
Prostadine for Prostate & Urinary Health?
Prostadine is a supplement that claims to support prostate health and bladder control, but does it really work? Find out in our Prostate Supplements Review, which includes our Top Pick among prostate supplements.
Also see our article about supplements to reduce nighttime urination.
Clinical Update
7/25/2023
Fenugreek Benefits?
Do fenugreek supplements lower blood sugar, increase testosterone, or have other potential health benefits? Find out in our new article about the health benefits and safety of fenugreek.
Clinical Update
7/28/2023
Toothpastes, Mouthwash, and Probiotics for Oral Health
We answered the following questions this week, all of which relate to our article about toothpastes and other dental products:
- Can hydroxyapatite toothpaste prevent cavities as effectively as fluoride toothpaste?
- Does chlorhexidine mouthwash affect blood pressure?
- Why do some toothpastes contain glycine, and is this ingredient beneficial?
- Are probiotics beneficial for oral health?
Also, find out the abrasiveness of two Fluoridex brand toothpastes.
Clinical Update
9/12/2023
Sea Moss
Sea moss, sometimes called Irish moss, is promoted for improving thyroid health, boosting immunity, and improving gut health, but are these uses supported by clinical evidence? Find out in our article about sea moss and its constituent carrageenan.
Clinical Update
1/17/2023
Tricaprin for Heart Health?
Is tricaprin (a medium chain triglyceride or MCT) beneficial in coronary artery disease and which products contain this ingredient? Find out in our updated CL Answer about supplements for heart health. (Also see our Review of MCT Oils.)
Clinical Update
8/26/2022
Lion's Mane for Depression, Anxiety, Gut Health
Does lion's mane (promoted for memory) help with depression, anxiety or gut health? See our article about lion's mane.
Clinical Update
3/01/2022
Glucosamine and Depression
Can taking glucosamine improve, or cause, depression? Find out in the Glucosamine and Chondroitin section of our Joint Health Supplements Review. Also see our Top Picks among joint health supplements.
Also see our answer to the question: What are the best supplements for depression and anxiety?
Clinical Update
2/23/2022
Coffee and Heart Health
Drinking moderate amounts of one type of coffee, but not another, was linked to a reduced risk of heart attack and stroke in a recent analysis. Get the details in our updated article about Coffee and Health.
Clinical Update
7/31/2025
Moringa for Prostate Health or Sleep?
Is there evidence that moringa leaf improves prostate health or sleep? Find out in our article about moringa supplements, which includes tips on choosing a moringa supplement.
Clinical Update
3/20/2025
Vanadium: Health Effects & Safety
Does vanadium, a trace mineral, have health benefits and is it safe? Find out in our article: What are trace minerals and do I need them?
Clinical Update
5/30/2025
Kirkland Joint Health Supplement Tested
Due to its popularity among CL members, we recently tested Kirkland Triple Action Joint Supplement, which contains a "proprietary" UC-II cartilage blend. See what we found in our Collagen Supplements Review, which includes our Top Pick for collagen for joints and for skin.
Also see our recent Joint Health Supplements Review, covering glucosamine, chondroitin, MSM, and Boswellia.
Clinical Update
2/06/2024
Coffee for Brain Health?
Find out if drinking coffee improves cognitive function and memory, affects the risk of Alzheimer's disease, and more in our article about the brain health benefits of coffee.
Clinical Update
12/16/2023
Does Milk Reduce Coffee Benefits?
Adding milk to coffee may potentially reduce coffee’s heart health benefits. Find out why in our article about Coffee and Heart Health.
Clinical Update
5/14/2024
Chlorophyll Supplements: Benefits and Risks
Do chlorophyll supplements reduce body odor, help prevent cancer, or have other health benefits, and are there adverse effects? Find out in our answer to the question: What are the health benefits of chlorophyll and chlorophyllin, and are they safe?
Clinical Update
5/21/2024
Andrographis Health Effects
Find out if andrographis helps with conditions such as cancer, COVID-19, and multiple sclerosis in our article about the health benefits and safety of andrographis.
Also see: Do any supplements reduce symptoms or relapse of multiple sclerosis?
Also see: Do any supplements help with COVID-19?
Clinical Update
6/04/2024
Caution With White Willow in Joint Supplements
A CL member asked about potential side effects of taking white willow. Be aware that white willow extract is added to some joint health supplements and can have side effects. For details, see the Concerns and Cautions section of our Joint Health Supplements Review, which includes our Top Picks among products.
Clinical Update
12/23/2024
Boswellia for Knee Osteoarthritis?
Did taking Boswellia extract (Aflapin/AprèsFlex — as found in Life Extension ArthroMax tested by ConsumerLab) reduce pain and improve function, joint space, and cartilage thickness in people with knee osteoarthritis? Find out what a recent study showed in the Boswellia section of our Joint Health Supplements Review, which includes our Top Pick for Boswellia and other joint health supplements.
Also see: Which supplements help with arthritis?
Clinical Update
2/27/2025
Walnuts for Heart Health?
Walnuts are permitted to bear a heart health claim – but it comes with a caveat. Get the details in our Shelled Walnuts Review, which includes our Top Picks among shelled walnuts, walnut halves & pieces, and chopped walnuts.
Clinical Update
11/11/2024
Joint Chew for Dogs?
Did a popular chew with Boswellia and collagen improve mobility or reduce joint pain among dogs with osteoarthritis? See what a recent study found in our Review of Joint Health Supplements for Pets, which includes our Top Picks among products. (Also see our Top Picks among joint health supplements for people.)
Learn more about ConsumerLab’s tests of supplements for pets.
Clinical Update
10/24/2024
Glucosamine Name Change
Following a legal settlement, a product tested in our Joint Health Supplements Review now identifies its glucosamine ingredient differently. For details, see the Update in the Review, which includes our Top Picks among joint health supplements.
Clinical Update
4/12/2024
Plant-Based Meat and Heart Health
Does replacing animal meat with plant-based “meat” such as the Beyond Burger or Impossible Burger improve heart health? Find out what a recent study showed in our article comparing the Beyond Burger, the Impossible Burger, and a beef burger.
Clinical Update
7/12/2024
Glucosamine & Cancer Risk?
Is the use of glucosamine associated with the risk of developing cancer? See what a recent study found in the What It Does section of our Joint Health Supplements Review. Also see our Top Picks for glucosamine and other joint health supplements.
Recalls & Warnings
April 02, 2021
Seller of Vision Supplements Warned by FDA
On March 19, 2021, the FDA issued a warning letter to Lipotriad LLC following a review of the company's websites, which found statements made about the company's products Lipotriad Visionary, Lipotriad Adult 50+, Lipotriad Dry Eye, and Lipotriad Vision Support Plus to ...
Recalls & Warnings
February 09, 2022
FDA Warns Seller of Colloidal Silver Eye Drops, Copper Products & More
On February 1, 2022, the FDA issued a warning letter to New Earth Healing Essentials, LLC d/b/a 5D Full Disclosure following a review of the company’s website, which found statements made about some of the company's products, including Plasma Colloidal Silver Eyedrops, Gaia’s ...
Recalls & Warnings
December 29, 2020
FDA Warns Five Sellers of CBD for Claims of Treating Arthritis, Alzheimer's and More
On December 22, 2020, the FDA issued warning letters to five sellers of CBD following reviews of the companies' websites, which found statements made about the companies' products to be drug claims.
Recalls & Warnings
March 11, 2024
Healthex Warned for Manufacturing Violations
On June 15, 2023, the FDA issued a Warning Letter to Healthex Distributors, Inc.
News Release
February 25, 2022
Top-rated Vitamin and Supplement Brands and Merchants for 2022 Based on Consumer Satisfaction
White Plains, New York, February 25, 2022 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 8,049 responses collected in November/December 2021.
Recalls & Warnings
March 30, 2021
Real Water Alkaline Water Recalled for Possible Link to Liver Illness
On March 24, 2021, Real Water, Inc. recalled all sizes of Real Water bottled alkaline water because it may be linked to multiple cases of non-viral hepatitis that occurred in Las Vegas, NV in November of 2020.
Recalls & Warnings
March 09, 2022
FDA Warns Seller of Magnesium, CBD, Herbal Extracts & More
On February 9, 2022, the FDA issued a warning letter to Bea Lydecker’s Naturals, Inc.
Recalls & Warnings
November 17, 2021
Mountain Meadow Herbs "Cleanse" Recalled, Risk of Injury from Exploding Bottle
On November 17, 2021, Mountain Meadow Herbs issued a recall of 54 bottles of Mountain Meadow Herbs brand Candida Flush due to the bottles becoming pressurized over time in storage.
Recalls & Warnings
August 07, 2023
Aerosol Bed Bug & Flea Killer Recalled Due to Injury Risk
On August 3, 2023, Maggie’s Farm issued a recall of the company’s Aerosol Bed Bug & Flea Killer products because the aerosol can can rupture and expel shrapnel, posing an injury and laceration hazard to consumers.
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
February 17, 2010
FTC Warns of Deceptive Claims with Children's Omega-3 Supplements
On February 17, 2010, the Federal Trade Commission (FTC) announced that it had sent letters to 11 companies that promote various omega-3 fatty acid supplements, telling them they should review their product packaging and labeling to make sure they do not violate federal law by making baseless ...
Recalls & Warnings
July 05, 2013
Seller of Vision Supplement Warned For Drug Claims
On June 25, 2013, the FDA issued a warning letter to Nutrient Synergy, Inc., following a review of the company's website which found statements made about the dietary supplement Nepretin to be drug claims.
Recalls & Warnings
March 17, 2015
Seller of Tea Warned for Drug Claims
On March 4, 2015, the FDA issued a warning letter to Four Elements Organic Herbals, LLC because statements made about HERBAL TEA Power, Energy & Stamina, ORGANIC HERBAL TEA Tulsi TelepaTea, ORGANIC HERBAL TEA To Your Health, Hawthorn Fresh Herb Extract, Elderberry Fresh Herb ...
Recalls & Warnings
March 06, 2015
Seller of Tea Drinks Warned for Drug Claims
On February 26, 2015, the FDA issued a warning letter to the seller of Anamu CancerHerb Tea Box, Anamu CancerHerb Tea Box with Honey and Organic Bottled Tea Drinks because statements made about these products on the websites, www.cancerherbtea.com and www.anamucancerherbtea.
Recalls & Warnings
February 17, 2015
Seller of Muscle Supplements Containing Synthetic Steroids Warned
On February 9, 2015, the FDA issued a warning letter to A2Z Industries, LLC, following a facility inspection which found the company's muscle enhancing supplements, including HaloV and EPI2A3A to be labeled as containing synthetic steroids.
Recalls & Warnings
February 06, 2015
Seller of Aloe Supplement Warned for Drug Claims
On January 21, 2015, the FDA issued a warning letter to Pristine Nutraceuticals, LLC, following a review of the company's website, http://www.digestaqure.com, which found statements made about DigestaCure to be drug claims.
Recalls & Warnings
March 18, 2021
Don't Drink Real Water Alkaline Water, FDA Warns After Reports of Liver Illness
On March 16, 2021, the FDA warned consumers not to drink or use Real Water bottled alkaline water while it investigates reports of hepatitis associated with the product.
Recalls & Warnings
July 21, 2022
UV Light Wands That May Cause Injury, According to the FDA
The FDA recently warned consumers of potential exposure to unsafe levels of ultraviolet-C (UV-C) radiation associated with the use of certain brands of ultraviolet (UV) wands, as found from testing conducted by the agency.
Recalls & Warnings
September 21, 2023
Moor Herbs, Inc Warned for Manufacturing Violations, Drug Claims
On August 1, 2023, the FDA issued a Warning Letter to Moor Herbs, Inc.
Recalls & Warnings
February 06, 2023
FDA Warns Companies Promoting Products to Treat Monkeypox, COVID-19, & Other Viruses
On January 30, 2023, the FDA issued a warning letter to four companies following review of the company websites, which found statements about company products to be drug claims because they were promoted to mitigate, prevent, treat, diagnose, or cure viruses such as monkeypox.
Recalls & Warnings
October 27, 2004
Some Supplements Can Damage Eyes At High Doses and with Topical Use
As reported by Reuters Health on October 21, 2004, an article in the current issue of the American Journal of Ophthalmology suggests that some herbal remedies and nutritional supplements can damage the eyes.
News Release
February 24, 2022
Consumers Returned to Pre-Pandemic Supplement Usage in 2021, ConsumerLab Survey Reveals
White Plains, New York, February 24, 2022 —A survey of 8,049 people who use dietary supplements shows many supplements that declined in use in 2020 began bouncing back in 2021, such as magnesium (+2.4 percentage points), and CoQ10 (+2.7 pts).
News Release
August 27, 2021
ConsumerLab Tests Reveal Best Joint Health Supplements for People and Pets
White Plains, New York, August 27, 2021 — Joint health supplements with ingredients such as glucosamine, chondroitin, MSM, boswellia, collagen and turmeric are often promoted to relieve joint pain or slow the progression of osteoarthritis.
Recalls & Warnings
May 15, 2016
Seller of Vision Supplements Warned for Drug Claims
On April 28, 2016, the FDA issued a warning letter to Macular Health, LLC.
Recalls & Warnings
March 10, 2015
Seller of Vision Supplements Warned for Manufacturing Violations, Drug Claims
On February 26, 2015, the FDA issued a warning letter to Biosyntrx, following a facility inspection which found the company's products, including BioTears Oral Gel Caps, ZoOmega-3 Concentrated Pharmaceutical-Grade Fish Oil, EpiCor A Nutrient-dense High Metabolite Immunogen, Sight C+ Mineral ...
News Release
May 18, 2021
Caution With Potassium Supplements: You Could Get More Than Expected
White Plains, New York, May 18, 2021 — Recent tests by ConsumerLab.com of popular potassium supplements on the market revealed that consumers to get much more or much less potassium than expected from certain products.
News Release
February 26, 2021
COVID Changed Supplement Popularity in 2020, ConsumerLab Survey Reveals
White Plains, New York, February 26, 2021 — A survey of 9,647 people who use dietary supplements shows that the supplements which experienced the greatest growth in popularity in 2020 were those being promoted to prevent or treat infection with SARS-CoV-2, the coronavirus that causes COVID-19.
News Release
February 25, 2021
Top-rated Vitamin and Supplement Brands and Merchants for 2021 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2021 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,647 responses collected in November 2020.
News Release
February 23, 2021
ConsumerLab Tests Reveal Best Prostate Supplements With Saw Palmetto and Beta-Sitosterol
White Plains, New York, February 23, 2021 — Over half of men over age 60 may experience increased urinary frequency, difficulty urinating, or other symptoms of an enlarged prostate, also known as benign prostate hyperplasia, or BPH.
News Release
January 16, 2021
Best Resveratrol Products Revealed in ConsumerLab Review
White Plains, New York, January 16, 2021 — Resveratrol supplements are promoted for a wide range of uses, including lowering cholesterol and improving cardiovascular health, improving memory, reducing inflammation and slowing age-related macular degeneration.
News Release
February 25, 2020
Top-rated Vitamin and Supplement Brands and Merchants for 2020 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2020 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,782 responses collected in late November and early December 2019.
News Release
November 12, 2019
Best Magnesium Supplements Revealed by ConsumerLab
White Plains, New York, November 12, 2019 — Many people do not get adequate amounts of magnesium from their diets, or may have lower levels of this essential mineral due to certain medical conditions or the use of common medications such as Nexium and Prilosec.
News Release
February 25, 2019
Top-rated Vitamin and Supplement Brands and Merchants for 2019 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
White Plains, New York, February 25, 2019 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,931 responses collected in late November and early December 2018.
News Release
January 15, 2019
Not All Lycopene Supplements Contain What They Claim, ConsumerLab Tests Reveal
White Plains, New York, January 15, 2019 — Foods rich in the antioxidant lycopene, like tomato juice and paste - may reduce the risk of certain cancers and have heart-health benefits, and preliminary studies suggest that lycopene supplements may also be helpful.
News Release
October 02, 2018
Best Joint Health Supplements Identified -- ConsumerLab Tests Glucosamine, Chondroitin, MSM and Boswellia Supplements for People and Pets
White Plains, New York, October 2, 2018 — Do joint health supplements with ingredients such as glucosamine, chondroitin, Boswellia, and MSM really relieve joint pain or slow the progression of osteoarthritis? If so, which products are best? To find out, ConsumerLab recently tested popular ...
News Release
February 25, 2018
Top-rated Vitamin and Supplement Brands and Merchants for 2018 Based on Consumer Satisfaction
White Plains, New York, February 25, 2018 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 11,446 responses collected in late November and early December 2017.
News Release
November 27, 2017
Flaxseed and Other Popular Omega 3 & 6 Oil Supplements Tested, But Not All Brands Pass -- Tests of Black Currant Oil, Borage Oil, Evening Primrose Oil, Flaxseed Oil, and Hemp Oil Supplements
White Plains, New York, November 27, 2017 — Flaxseed oil and other popular seed oil are popular supplements that provide omega-3 and omega-6 fatty acids and may help with inflammatory conditions. But which seed oil supplements are the best? To find out, ConsumerLab.
News Release
February 25, 2017
Top-rated Vitamin and Supplement Brands and Merchants for 2017 Based on Consumer Satisfaction
White Plains, New York, February 25, 2017 — Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 9,505 responses collected in late November and early December 2016.
News Release
September 13, 2016
Best Fish Oil? ConsumerLab.com Tests 44 Popular Omega-3 Supplements and Identifies Its Top Picks
White Plains, New York, September 13, 2016 — Fish oil and omega-3 fatty acid supplements containing EPA and DHA are among the most popular supplements, and clinical studies suggest a wide range of potential benefits, from improving mental health to treating inflammatory disease.
News Release
June 03, 2016
What Works for Joint Health? ConsumerLab.com Reviews Glucosamine, Chondroitin, MSM and Boswellia Supplements for People and Pets
White Plains, New York, June 3, 2016 — Do popular joint health supplements with ingredients such as glucosamine, chondroitin, Boswellia, and MSM really work to relieve pain or slow the progression of osteoarthritis? And, with so many to choose from, which supplements are best? To find out, ...
News Release
February 25, 2016
Top-rated Vitamin and Supplement Brands and Merchants for 2016 Based on Consumer Satisfaction
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 11,534 responses collected in November, 2015. Respondents gave ratings for 963 brands and 399 merchants.
Recalls & Warnings
February 19, 2005
Seller of Eye Supplement Purporting to Restore Vision and Eliminate "Floaters" Settles FTC Charges
On February 15, 2005, the Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, have settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular ...
News Release
December 17, 2015
ConsumerLab.com Focuses on Vision Supplements, Spotting the Best and the Worst
White Plains, New York, December 17, 2015 — Supplements containing lutein and other ingredients have been shown to slow deterioration of the retina in people with age-related macular degeneration.
Recalls & Warnings
September 17, 2025
Bariatric Fusion Multivitamins Recalled
On September 11, 2025, the Consumer Product Safety Commission and Blueroot Health issued a recall of several lots of Bariatric Fusion High ADEK multivitamin capsules because they contain a significant amount of iron (45 mg) but are not packaged with child-resistant bottle caps.
News Release
April 28, 2015
Best Supplements for Bone Health? ConsumerLab.com Finds Products with Multiple Ingredients More Likely to Contain Wrong Amounts
White Plains, New York, April 28, 2015 — Vitamin D and calcium each play a crucial role in bone health and are among the most popular supplement ingredients in the U.S., but recent ConsumerLab.
News Release
February 25, 2015
Top-rated Vitamin and Supplement Brands and Merchants for 2015 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,329 responses collected in November, 2014. Respondents gave ratings for 1,709 brands and 891 merchants.
News Release
December 10, 2014
ConsumerLab.com Identifies Best Zinc Products for Colds, Eyes, and Zinc Deficiency -- Popular Zinc Supplements and Lozenges Tested and Compared
White Plains, New York, December 10, 2014 — "Zinc supplements have been shown to shorten colds and reduce the progression of advanced macular degeneration, but not all products provide the dosage proven effective, as well as pass criteria for supplement quality," says Tod Cooperman, M.D.
News Release
April 09, 2014
Best Fish Oil? ConsumerLab.com Testing Finds Many High Quality Supplements, But Pitfalls Exist
White Plains, New York, August 10, 2014 — Recent laboratory tests of fish oil, krill oil, algal oil, calamari oil, and green-lipped mussel oil supplements by ConsumerLab.com revealed quality problems with four out of 30 supplements selected for review.
News Release
February 26, 2014
Mislabeling of Some Joint Health Supplements with Glucosamine, Chondroitin, Boswellia, and MSM According to ConsumerLab.com -- 38 Supplements Tested, Including Products for Dogs and Cats --
White Plains, New York, February 26, 2014 — Glucosamine, chondroitin, Boswellia, and MSM are popular dietary supplement ingredients for treating symptoms of osteoarthritis — worn joint cartilage.
News Release
February 12, 2014
Top-rated Vitamin and Supplement Brands and Merchants for 2014 Based on Consumer Satisfaction -- Results of the ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,326 responses collected in November, 2013. Respondents gave ratings for 1,639 brands and 788 merchants.
News Release
December 16, 2013
What's Really in Turmeric Supplements and Spices? You May Not Want to Know -- 33% of Turmeric/Curcumin Supplements Fail ConsumerLab.com's Review; Filth Found in Spices
White Plains, New York, December 16, 2013 — Supplements containing the herb turmeric or its key constituent, curcumin, are popular treatments for inflammatory diseases. ConsumerLab.
News Release
October 17, 2012
ConsumerLab.com Tests and Compares Vision Supplements - Guides Consumers Through a Variety of Ingredients and Formulas
White Plains, New York — October 17, 2012 — Can a supplement improve your vision or prevent it from deteriorating? "The evidence shows that some supplements may help improve vision in people with macular degeneration and others can slow its progression," says Tod Cooperman, M.D.
News Release
August 22, 2012
Contamination and Other Problems Found in Fish Oil Supplements -- Large Review by ConsumerLab.com Reveals Excessive PCBs or Mislabeling in Over 30% of Omega-3 Supplements
August 22, 2012 — The quality of fish oil / omega-3 supplements varies across brands, according to recent tests by ConsumerLab.com, which independently reports on the quality of health products. Analyses of 35 products selected and purchased by ConsumerLab.
News Release
May 08, 2012
Lead Contamination and Mislabeling in Joint Health Supplements with Glucosamine, Chondroitin, and MSM According to ConsumerLab.com
White Plains, New York, May 8, 2012 — Glucosamine, chondroitin, and MSM are popular dietary supplements for treating symptoms of osteoarthritis — worn joint cartilage.
News Release
April 26, 2012
ConsumerLab.com reviews prostate supplements with saw palmetto and/or beta-sitosterol and finds few likely to be effective
White Plains, New York April 26, 2012 — Can supplements reduce the symptoms of prostate enlargement in men, as some products claim? ConsumerLab.com researchers recently tackled this question, investigating the evidence behind prostate supplements and testing the quality of these products.
News Release
February 01, 2012
Top-rated vitamin and supplement brands and merchants on consumer satisfaction for 2012 -- Based on ConsumerLab.com Survey of Vitamin & Supplement Users
Each year, ConsumerLab.com surveys its free e-newsletter subscribers about the vitamins and supplements that they use. The results below are based on 10,260 responses collected in November, 2011. Most respondents used multiple supplements.
News Release
December 20, 2011
Choose zinc supplements carefully -- ConsumerLab.com finds only some have dosage proven to shorten colds, reduce eye disease
White Plains, New York — December 20, 2011 — Can zinc supplements shorten colds and reduce the progression of advanced macular degeneration? "Yes, but not all supplements provide a dosage that has been proven effective." says Tod Cooperman, M.D., President of ConsumerLab.com.
News Release
September 21, 2011
Not all vitamin A supplements pass ConsumerLab.com tests -- New report reviews beta-carotene and cod liver oil supplements
White Plains, New York — September 21, 2011 — Recent tests of vitamin A supplements by ConsumerLab.com found one to contain only 54.3% of its listed amount of vitamin A. Another product mislabeled alpha carotene as vitamin A.
News Release
February 16, 2011
Some turmeric and curcumin supplements fail quality review -- ConsumerLab.com finds 20% of turmeric supplements selected for review deliver less than 15% of promised compounds
WHITE PLAINS, NEW YORK — FEBRUARY 16, 2011 — Supplements containing the herb turmeric or its key compound, curcumin, have become popular in the U.S. ConsumerLab.com cautioned today that two out of ten turmeric products recently selected for quality testing were found to provide only 7.
News Release
December 16, 2009
"Eye health" supplements vary widely in formula but product quality is high according to ConsumerLab.com
"Eye Health" Supplements Vary Widely in Formula But Product Quality is High According to ConsumerLab.com
News Release
July 10, 2009
Nine joint health supplements fail ConsumerLab.com quality review -- Lead contamination, missing ingredients and other problems found in supplements for people as well as for dogs, cats and horses; Many others approved
WHITE PLAINS, NEW YORK — JULY 10, 2009 — Nine supplements for osteoarthritis that ConsumerLab.com recently selected for testing were found to be contaminated with lead, lack all or some of a key ingredient or have other quality problems.
News Release
March 10, 2009
ConsumerLab.com reports deficiencies in some supplements containing calcium, vitamin D, or vitamin K -- Focus on bone health supplements and new uses for vitamins D and K
WHITE PLAINS, NEW YORK — MARCH 10, 2009 — In a large review of supplements relating to bone health, ConsumerLab.com reported today that tests showed five products to provide only 52.5% to 89% of the listed amounts of calcium or vitamin K. ConsumerLab.
Recalls & Warnings
June 06, 2017
Seller of Vision, Cholesterol, Prostate Supplements and More Warned for Drug Claims
On May 25, 2017, the FDA issued a warning letter to Herbal Doctor Remedies following a facility inspection and review of the company's websites, www.herb-doc.com, www.ChinaSecretMed.com and www.theraherb.
News Release
December 09, 2008
Tests of zinc supplements by ConsumerLab.com show only some provide dosage proven to shorten colds, reduce eye disease. Lead contamination found in one product.
WHITE PLAINS, NEW YORK — DECEMBER 9, 2008 — Zinc supplements can shorten colds and reduce the progression of advanced macular degeneration, among other uses. But a new report by ConsumerLab.
News Release
November 18, 2008
Adulteration Suspected with Some "Memory" Supplements -- Few Ginkgo and Huperzine Supplements Pass ConsumerLab.com Tests; Quality High for Acetyl-L-Carnitine
WHITE PLAINS, NEW YORK — NOVEMBER 18, 2008 — Tests by ConsumerLab.com of Ginkgo biloba supplements show that few products meet quality standards.
News Release
October 28, 2008
Tests reveal quality problems with vitamin A supplements -- New ConsumerLab.com report focuses on vitamin A, including beta-carotene and cod liver oil
WHITE PLAINS, NEW YORK — OCTOBER 28, 2008 — ConsumerLab.com is cautioning that the amount vitamin A in some supplements may be much higher or lower than the amount stated on labels. Among ten supplements recently selected for testing, ConsumerLab.
News Release
July 12, 2007
ConsumerLab.com finds lutein & zeaxanthin supplements vary widely in strength but most meet ingredient claims — Right amount of antioxidant may help prevent macular degeneration and improve vision
WHITE PLAINS, NEW YORK — July 12, 2007 — ConsumerLab.com announced today that all but one of the thirteen lutein and zeaxanthin supplements it recently chose to test met quality standards.
News Release
June 12, 2007
Tests show many milk thistle supplements low in key component — Seven products fail ConsumerLab.com testing of herb used in diabetes and liver diseases
WHITE PLAINS, NEW YORK — June 12, 2007 — Tests by ConsumerLab.com of nine milk thistle supplements that it selected showed that only two contained the expected amounts of silymarin compounds, believed to be the active constituent of milk thistle.
News Release
May 15, 2007
ConsumerLab.com finds some alpha lipoic acid supplements come up short — Improper formulation may cause ingredient degradation
WHITE PLAINS, NEW YORK — May 15, 2007 — ConsumerLab.com announced that two of eleven alpha lipoic acid supplements that it recently chose to test contained significantly less ingredient than promised on their labels.
News Release
April 24, 2007
ConsumerLab.com reports improvement in quality of SAM-e supplements used for osteoarthritis and depression — Recent testing shows quality is up since studies in 2000 and 2003
WHITE PLAINS, NEW YORK — April 24, 2007 — ConsumerLab.com announced that all eight SAMe supplements that it recently selected for review passed quality testing — an improvement over tests in previous years. SAMe (S-adenosyl-methionine) is used to treat depression and joint pain.
News Release
April 11, 2007
Many arthritis supplements lack key listed ingredient — ConsumerLab.com finds chondroitin missing or low in eight supplements; Glucosamine and MSM also tested
WHITE PLAINS, NEW YORK — April 11, 2007 — Forty percent of the supplements for osteoarthritis that ConsumerLab.com recently selected for testing were found to lack all or some of their key ingredients. Osteoarthritis is the most common type of arthritis, affecting an estimated 20.
News Release
March 06, 2007
ConsumerLab.com produces series of free online seminars about dietary supplements — First seminar on "joint health" now available
WHITE PLAINS, NEW YORK — MARCH 6, 2007 — ConsumerLab.com announced today that it is producing and webcasting a series of free, online audio seminars, or "webinars," that explain the use of popular types of dietary supplements.
News Release
February 14, 2007
ConsumerLab.com reports on anti-depressant herb — St. John's Wort — Few supplements pass quality testing
WHITE PLAINS, NEW YORK — FEBRUARY 14, 2007 — St. John's wort may be helpful in treating mild to moderate cases of clinical depression, but a new report from ConsumerLab.com shows that few of the herbal products tested met quality standards. Americans purchased $64 million of St.
News Release
January 19, 2007
Consumers warned of problems with multivitamins — ConsumerLab.com uncovers defects in over half of products tested
WHITE PLAINS, NEW YORK — JANUARY 19, 2007 — Fifty-two percent of multivitamins recently selected for testing by ConsumerLab.com were found to be contaminated with lead, unable properly break apart, or to contain significantly more or less ingredient than claimed.
News Release
December 12, 2006
Probiotic supplements grow in popularity but viable bacteria missing in many — ConsumerLab.com cautions consumers to select probiotics carefully and store them properly
WHITE PLAINS, NEW YORK — DECEMBER 12, 2006 — Sales of supplements containing beneficial organisms (probiotics) have grown rapidly, but a new test report from ConsumerLab.com shows that 44% contained fewer viable organisms than claimed or generally known to be effective.
News Release
November 13, 2006
Tests of "muscle" supplements finds some "weak" products but most contain expected creatine, HMB, or amino acids — Review of muscular enhancement supplements published by ConsumerLab.com
WESTCHESTER, NEW YORK — MONDAY, NOVEMBER 13, 2006 — Bodybuilders and athletes often turn to supplements to enhance muscle size and strength.
News Release
March 13, 2006
ConsumerLab.com reports on supplements for bone health containing calcium and vitamin D— Results posted for 32 supplements for adults and children; One found contaminated with lead
WESTCHESTER COUNTY, NEW YORK — MARCH 13, 2006 — ConsumerLab.com announced test results today from its new Product Review of Supplements for Bone Health covering 32 adult and children's products containing calcium and vitamin D. Sales of calcium supplements in the U.S.
News Release
December 14, 2004
ConsumerLab.com expands testing to Japan — Testing Organization Expands to Help Japanese Consumers Determine Quality of Health and Nutrition Products
WHITE PLAINS, NY — Tuesday, December 14, 2004 — ConsumerLab.com announced today that it would begin testing products sold in Japan. Its first report is due out in early spring. In the U.S. and Canada, ConsumerLab.
News Release
August 25, 2004
ConsumerLab.com finds not all vitamin E products meet claims — Results for 33 vitamin E supplements and skin products released today
WHITE PLAINS, NY — Wednesday, August 25, 2004 (Updated 10/24/04) — ConsumerLab.com announced today that five vitamin E products failed to pass recent testing for having either too little vitamin E and/or for containing synthetic vitamin E when claiming to be natural.
News Release
March 02, 2004
ConsumerLab.com survey finds most consumers highly satisfied with supplements; Ratings vary by brand
WHITE PLAINS, NY — March 2, 2004 — ConsumerLab.com announced today that 78% of consumers recently surveyed report being "extremely" or "very" satisfied with the supplement brands they used. Ratings varied by brand. The highest rating was for Nutrilite (96%), a direct sales brand.
News Release
January 29, 2004
ConsumerLab.com finds lutein supplements vary widely in strength but most meet ingredient claims — Right amount of antioxidant compound may reduce risk of blindness
WHITE PLAINS, NY — January 29, 2004 — ConsumerLab.com announced today that only one of the 19 lutein/zeaxanthin supplements it recently tested failed to contain the claimed ingredients. Although product quality was generally high, ConsumerLab.
News Release
January 13, 2004
ConsumerLab.com finds discrepancies in strength of CoQ10 supplements — Increasingly popular supplement, but health professionals and consumers cautioned to check brands
WHITE PLAINS, NY — January 13, 2004 — (Updated January 30) — ConsumerLab.com today announced that among the coenzyme Q10 (CoQ10) supplements it recently tested there was no detectable CoQ10 in one product.
News Release
August 28, 2003
Some problems persist with ginseng supplements but overall quality improves according to ConsumerLab.com — Test results published online today
WHITE PLAINS, NY — August 28, 2003 — In contrast to its testing three years ago in which nearly 60% of Asian and American Ginseng supplements were found to have significant problems, testing of recently purchased products by ConsumerLab.
News Release
July 10, 2002
Pharmavite dietary supplements receive ConsumerLab.com approval — Vitamin E, SAM-e, St. John's Wort, Ginkgo and others merit approved quality products ranking
WHITE PLAINS, NY — July 10, 2002 — ConsumerLab.
News Release
May 05, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 5, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
Recalls & Warnings
March 17, 2025
Zaarah Herbal Powders Recalled Due to Lead, Arsenic Risk
On March 10, 2025, New York Wholesale Group recalled Zaarah Herbals Rasayan Churan, Zaarah Herbals Gurmar Powder, Zaarah Herbals Vasaka Powder and Zaarah Herbals Bhringraj Powder because they have the potential to be contaminated with elevated levels of lead and arsenic.
Recalls & Warnings
May 09, 2025
Organic Traditions Pumpkin Seeds Recalled in U.S. and Canada
On May 8, 2025, Advantage Health Matters Inc. recalled packages of Organic Traditions Organic Jumbo Pumpkin Seeds in the U.S. and Canada because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
November 18, 2024
Manufacturer of Grandma’s Herbs Kidney Warned by FDA
On October 20, 2023, the FDA issued a warning letter to Top Health Manufacturing, LLC, manufacturer of Grandma’s Herbs Kidney supplement, following an inspection of the company’s facility that found its products to be adulterated because they were, packed, labeled or held under ...
News Release
March 06, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 6, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
News Release
August 05, 1999
ConsumerLab.com initiates independent testing of herbal products and supplements. Certification Seal announced.
WHITE PLAINS, NY, August 5, 1999 — ConsumerLab.com, LLC announced that it has begun a large-scale program of independent testing of herbal products and dietary supplements. .
Recalls & Warnings
May 20, 2005
Seller of "Ocular Nutrition" Supplement Settles FTC Charges
On March 16, 2005, The Federal Trade Commission (FTC) announced that Hi-Health Supermart Corporation (Hi-Health) and its owner, Simon Chalpin, settled FTC charges that they made unsubstantiated advertising claims that their product – Premier Formula for Ocular Nutrition-Optim3 (Ocular Nutrition) – ...
Recalls & Warnings
April 30, 2021
FDA Warns Seller of Fish Oil, Apple Cider Vinegar, Collagen, and Elderberry Supplements
On April 13, 2021, the FDA issued a warning letter to Orphic Nutrition following a review of the company's websites, which found statements made about the products Apple Cider Vinegar Capsules, Ashwagandha Capsules, Garcinia Cambogia Capsules, Premium Collagen Peptides Capsules, Elderberry ...
Recalls & Warnings
July 28, 2010
Choking Concern Prompts Eye Supplement Recall
On July 28, 2010, the U.S. FDA posted a recall by Bausch + Lomb of its PreserVision® Eye Vitamin AREDS 2 Formula with Omega 3 soft gels, only available within the United States.
Recalls & Warnings
January 04, 2020
Hormonal Balance Supplement Tied to Liver Failure
An otherwise healthy 23-year old woman in Texas is reported to have recently developed liver failure (requiring a liver transplant) after taking the supplement Balance (from Alani Nu) for four months.
Recalls & Warnings
January 04, 2024
Lone Star Botanicals Warned by FDA for Manufacturing Violations
On November 26, 2023, the FDA issued a Warning Letter to Lone Star Botanicals, Inc.
Recalls & Warnings
April 25, 2024
Drug Found in Umary Supplement
On April 18, 2024, CTV News in Canada reported tests suggesting that Umary Hyaluronic Acid Dietary Supplement may be adulterated with the prescription anti-inflammatory drug diclofenac, which may explain side effects that have been reported with its use.
Recalls & Warnings
February 11, 2012
Herbal Sprays for Colds and Cold Sores Recalled
On February 10, 2012 the U.S. FDA posted a notice announcing the recall of all "Koff & Kold" and "Kold Sore" sprays by Wholistic Herbs Inc. The products were distributed throughout the United States.
Recalls & Warnings
December 22, 2005
Health Canada Warns Consumers Not to Take Chaparral
On December 21, 2005, Health Canada (Canada's health ministry) warned consumers not to ingest the herb chaparral in the form of loose leaves, teas, capsules or bulk herbal products because of the risk of liver and kidney problems.
Recalls & Warnings
May 01, 2023
TruVision Recalls Products Due to Presence of Potentially Dangerous Ingredients
On April 27, 2023, TruVision Health issued a recall of various dietary supplement products because they contain hordenine and/or octodrine/DMHA, compounds that the FDA considers to be “possibly unsafe” and which are not permitted to be sold as dietary supplements.
Recalls & Warnings
October 18, 2017
FDA Warns Seller of Arthritis, Blood Pressure, Diabetes Supplements For Making Drug Claims
On August 15, 2017, the FDA issued a warning letter to Years to Your Health, Inc., following an inspection of the company's website which found a number of their products to be misbranded, as they contained drug claims.
Recalls & Warnings
November 21, 2017
Health Canada Calls for Stronger Warnings of Liver Risk on Green Tea Extract Products
On November 15, 2017, Health Canada (the Canadian equivalent of the FDA) recommended stronger warning statements about the risk of liver toxicity on labels of supplements containing green tea extract sold in Canada.
Recalls & Warnings
January 02, 2004
Canada Reiterates Warning on Products Containing Kava
On December 23, 2003, Health Canada reminded Canadian consumers of the serious risks associated with the use of products containing kava.
Recalls & Warnings
May 01, 2009
FDA Warns Consumers to Stop Using Hydroxycut -- Product Being Tested by ConsumerLab.com
On May 1, 2009, the U.S. Food and Drug Administration warned consumers to immediately stop using Hydroxycut products by Iovate Health Sciences Inc., of Oakville, Ontario and distributed by Iovate Health Sciences USA Inc. of Blasdell, N.Y.
Recalls & Warnings
January 27, 2015
Seller of Aloe, Sexual Enhancement Supplements and More Warned for Manufacturing Violations, Drug Claims
On January 8, 2015, the FDA issued a warning letter to Aloe Man, Inc., following a facility inspection which found the company's products, including The Aloe Man's Super Bright, Dr. Johnson's Maximum Desire, Dr. Johnson's Body Healer, and Dr.
Recalls & Warnings
March 28, 2011
Dangerously High Levels of Vitamins A and D in Product Prompt FDA Warning
On March 28, the U.S. FDA warned consumers to stop using Soladek, a vitamin-solution product marketed by Indo Pharma, S.A., of the Dominican Republic, because the product may contain dangerously high levels of vitamins A and D.
Recalls & Warnings
January 07, 2002
Liver Toxicity with Kava
As announced in a December 19, 2001 letter to healthcare professionals, The Food and Drug Administration (FDA) is investigating whether the use of dietary supplements containing kava (also known as kava kava or Piper methysticum) is associated with liver toxicity.
Recalls & Warnings
July 29, 2009
FDA Warns Against Body Building Supplements with Steroid-like Compounds
On July 28, 2009, the U.S. FDA notified the public about new safety information concerning products marketed for body building and increasing muscle mass.
Recalls & Warnings
April 24, 2023
FTC Warns Almost 700 Companies: Unsupported Health Claims May Result in Substantial Civil Penalties
On April 13, 2023, the Federal Trade Commission (FTC) notified approximately 670 companies that market over-the-counter (OTC) drugs, homeopathic products, dietary supplements, or functional foods that they could incur significant civil penalties if they make unsubstantiated claims about the ...
Recalls & Warnings
March 22, 2023
Of Concern: The Daily Post and Hiya
White Plains, New York, March 22, 2023 — An "advertorial" for Hiya Kids Daily Multivitamin appearing on the website "The Daily Post" provided misinformation and suggested that ConsumerLab.com recommended this product, which is completely false.
Recalls & Warnings
May 08, 2023
SD Biosensor Pilot At-Home COVID-19 Tests Recalled Due to Bacterial Contamination
On May 4, 2023, the FDA warned consumers and health care providers not to use certain lots of Roche Diagnostics’ SD Biosensor, Inc. Pilot COVID-19 At-Home Tests due to bacterial contamination in the test kit’s liquid solution.
Recalls & Warnings
November 28, 2022
Seller of Joint Health, Collagen Protein and More Warned for Drug Claims
On November 14, 2022, the FDA issued a warning letter to The Truth Company, LLC (parent company of Kinobody, LLC and UMZU, LLC) following inspection of the company’s websites which found statements about its Betaine, Immune, Redwood, Sensolin, Thyrite, zuRelief, Kino Aminos, Kino Collagen ...
Recalls & Warnings
January 02, 2004
Health Canada Warns Consumers Not to Use Health Products Containing Comfrey
On December 12, Health Canada advised Canadian consumers not to ingest the herb comfrey or any health products that contain comfrey because they might contain a compound called echimidine, which may cause liver damage.
Recalls & Warnings
August 25, 2002
Canadian Recall and Stop-Sale Order on All Kava Products
Health Canada is issuing a stop-sale order for all products containing the herb kava after a safety assessment concluded there is insufficient evidence to support their safe use. The department is also requesting the recall of these products from all levels of the market.
Recalls & Warnings
January 29, 2004
U.S. Action on Mad Cow -- Supplements Included As Well as Food
On Jan. 26, 2004, the U.
Recalls & Warnings
February 20, 2013
Cold, Flu and Stress Supplement Company Warned For Adulteration, Drug Claims And More
On October 5, 2012, the FDA issued a warning letter to dietary supplement manufacturer Wholistic Herbs, Inc. following a facility inspection which found the company's products, including At-Ease, Morning Calm, Aller-Ban, Stomach Flu, Kold & Koff, Kold Sore, and L.
Recalls & Warnings
February 21, 2013
Pet Supplement Company Issues Recall Due To Potential Bacterial Contamination
On February 20, 2013, animal supplement company Nutri-Vet, LLC issued a recall of Nutri-Vet and NutriPet Chicken Jerky Products because they may be contaminated with Salmonella.
Recalls & Warnings
August 27, 2013
FTC Refunds Consumers of Children's Vitamins as Part of Deceptive Advertising Settlement
On August 8, 2013, the Federal Trade Commission (FTC) announced it has mailed 10,144 refund checks to consumers who purchased Disney or Marvel Hero -themed children's vitamins, including Disney Princesses, Winnie the Pooh, Finding Nemo, and Spider-Man varieties, between May 1, 2008 and September ...
Recalls & Warnings
October 09, 2013
FDA Warns Consumers Not to Use OxyElite Pro As It Investigates Link With Hepatitis
On October 8, 2013, the FDA warned consumers not to buy or use USPLabs’ weight loss supplement OxyElite Pro because it has been linked with 24 cases of acute non-viral hepatitis in Hawaii.
Recalls & Warnings
November 04, 2016
Energy Drink Linked to Case of Acute Hepatitis
A case of acute hepatitis has been linked to daily consumption of energy drinks, according to a report published on November 1, 2016, in BMJ Case Reports.
Recalls & Warnings
June 05, 2018
FDA Warns Seller of Colloidal Silver
On May 17, 2018, the FDA issued a warning letter to Silver Armor, Inc.
Recalls & Warnings
March 25, 2002
FDA Advises Consumers of Risk of Severe Liver Toxicity with Kava
On March 25, 2002 the U.S. Food and Drug Administration (FDA)issued a Consumer Advisory, as well as a letter to health care providers, advising of the potential risk of severe liver injury associated with the use of kava-containing dietary supplements.
Recalls & Warnings
November 21, 2014
Seller of Diabetes, Prostate Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 7, 2014, the FDA issued a warning letter to dietary supplement distributor Windmill Health Products, LLC, following a facility inspection which found the company's products, including Nutri-Betic caplets, Vita-betic caplets, ProstrinRx tablets, Polyflavanol capsules, and Glucoflex Joint ...
Recalls & Warnings
March 02, 2022
Seller of Holistic Tea Warned by FDA
On February 17, 2022, the FDA issued a warning letter to Vital Health & Wellness following an inspection of the company’s website, which found statements made about the company’s product, Dr. Miller’s Holistic Premium Holy Tea, to be drug claims.
Recalls & Warnings
March 09, 2022
Court Bars Salud Natural From Selling Aloe, Joint Supplements & More
On March 8, 2022, a federal court ordered Salud Natural Entrepreneur, Inc. to stop distributing nutritional supplements that violate the Federal Food, Drug and Cosmetic Act (FDCA).
Recalls & Warnings
August 08, 2022
Woman Pleads Guilty for Selling and Promoting Products to Treat COVID-19
On July 27, 2022, Diana Daffin, owner of Savvy Holistic Health pleaded guilty to selling products with claims they could treat COVID-19 and promised “Immunity for Humans.” The products were sold under the HAMPL brand name.
Recalls & Warnings
September 15, 2022
FDA Warns Parents Not to Use Mother's Touch Infant Formula
On September 6, 2022, the FDA warned parents and caregivers not to buy or give Mother’s Touch Formula to infants.
Recalls & Warnings
July 06, 2022
FDA Warns Kratom Companies for Arthritis, Pain Relief Claims & More
On January 30, 2022, the FDA issued warning letters to four sellers of kratom products because they were promoted to treat pain relief, opioid withdrawal, blood sugar control, anxiety, and to treat rheumatoid arthritis.
Recalls & Warnings
July 06, 2022
Two Companies Banned From Selling Supplements to Treat Heart Disease, Neuropathy
On June 30, 2022, the Federal Trade Commission (FTC) finalized an administrative complaint order against two Texas-based companies, Health Research Laboratories, LLC and Whole Body Supplements, LLC, for making unverified claims that their products can prevent or treat disease.
Recalls & Warnings
February 15, 2006
New Zealand Warns of Liver Toxicity from Black Cohosh
On February 9, 2006, New Zealand's Therapeutic Goods Administration (TGA) announced new labelling and consumer information for medicines containing Black cohosh (Cimicifuga racemosa).
Recalls & Warnings
April 02, 2021
FDA Warns Sellers of Prostate, Reishi, Immune Products, and More
On March 16, 2021, the FDA issued warning letters to two companies following reviews of their websites which found statements made about the companies' products to be drug claims.
Recalls & Warnings
November 20, 2020
FTC Files Complaint Against Two Supplement Companies for Deceptive Marketing
On November 20, 2020, the FTC approved a Part 3 administrative complaint against Health Research Laboratories, LLC, its owner Kramer Duhon, and Whole Body Supplements, LLC for making unverified claims that their products can prevent or treat diseases.
Recalls & Warnings
May 29, 2021
FDA, FTC Warns Five Sellers of "Infertility" Supplements
The FDA and FTC (Federal Trade Commission) sent warning letters to the following five companies in May for illegally selling dietary supplements promoted with claims to treat infertility and other reproductive health issues:
Recalls & Warnings
November 24, 2020
Recalled Vitamin D Contains Potentially Dangerous Ingredient
On November 22, 2020, Fusion Health and Vitality LLC recalled all 2020 lots of CORE Essential Nutrients and Immune Boost Sublingual Vitamin D3 because they are adulterated. CORE Essential Nutrients contains the unapproved food additive hordenine HCl.
Recalls & Warnings
March 05, 2021
FTC Takes Further Action Against Deceptive CBD Claims
On March 5, 2021, the Federal Trade Commission (FTC) announced that it has approved final administrative consent orders against six companies for selling CBD products with unsupported and deceptive health claims that they can treat a variety of conditions, including cancer, heart disease, ...
Recalls & Warnings
January 21, 2021
Federal Court Bars Fusion Health From Promoting Vitamin D for COVID-19
On January 8, 2021, the United States Department of Justice announced a permanent injunction has been entered, barring dietary supplement marketer Matthew Ryncarz and his companies Fusion Health and Vitality LLC dba Pharm Origins and Fusion Ionz LLC dba Pharm Origins from making claims that their ...
Recalls & Warnings
December 31, 2020
FDA Warns Seller of Liposomal Vitamin C, Vitamin D & More
On December 21, 2020, the FDA issued a warning letter to Sparrow Health & Performance LLC for selling the products Organic Liposomal Vitamin C, Nanoemulsified D3K2 (also called Liquid Liposomal Vitamin D3 with K2 or Liposomal Vitamin D3) and Immune Support ...
Recalls & Warnings
December 11, 2020
Seller of "Dr. Hotze's Immune Pak" Products Warned for COVID-19 Claims
Seller of "Dr. Hotze's Immune Pak" Products Warned for Coronavirus Claims
Recalls & Warnings
September 17, 2003
FDA Warns of Illness from Star Anise Teas
On September 10, 2003, the Food and Drug Administration (FDA) advised consumers not to consume "teas" brewed from star anise.
Recalls & Warnings
March 20, 2020
Seller of Rejuvenation Pills Settles Charges of Making False Claims
Health Center, Inc. has agreed to halt their allegedly deceptive advertising claims about three "cure-all" health and wellness products that targeted older consumers nationwide after the Federal Trade Commission (FTC) filed a complaint.
Recalls & Warnings
April 17, 2020
Joint Pain Supplement Isoprex Settles Charges of Making False Claims
On April 16, 2020, Renaissance Health Publishing, LLC, agreed to halt their allegedly deceptive advertising claims about their Isoprex supplement that targeted older consumers nationwide after the Federal Trade Commission (FTC) filed a complaint.
Recalls & Warnings
May 09, 2020
FTC Warns 45 More Companies for Coronavirus Claims
On May 7, 2020, the FTC announced that it sent warning letters to 45 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
September 08, 2020
Holistic Healthy Pet Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Savvy Holistic Health dba Holistic Healthy Pet following a review of the company's website, which found statements made about some of the company's products, including Doctor's Best for Your Pets, Savvy Holistic Health Pet Essences, ...
Recalls & Warnings
March 13, 2020
FDA Finds Problems at 52% of Supplement Manufacturing Sites in U.S. and 42% Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2019 (October 1, 2018 - September 30, 2019) of 598 dietary supplement manufacturing facilities in the U.S.
Recalls & Warnings
October 21, 2014
Seller of Weight Loss, Saw Palmetto Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Bethel Nutritional Consulting, Inc., following a review of the company's website which found statements made about 15 Day Detox Diet, Alpha Lipoic Acid. 300 mg.
Recalls & Warnings
July 12, 2015
Marketers of Memory Supplement to Pay $1.4 Million to Settle FTC Charges
On July 8, 2015, the FTC announced the marketers of Procera AVH (Brain Research Labs Inc.) have agreed to pay $1.4 million to settle charges they made deceptive claims that the supplement could significantly improve memory, mood and cognitive function.
Recalls & Warnings
November 20, 2013
OxyElite Pro Recall Expanded
On November 19, 2013, USPlabs LLC expanded its recall of OxyElite Pro products to include Raspberry Lemonade OxyELITE Pro Super Thermo Powder (4.6 oz., UPC #094922447494).
Recalls & Warnings
May 03, 2013
Homeopathic Company Warned For Drug Claims and Improper Labeling
On March 12, 2013, the FDA issued a warning letter to Natural Medicine Associates, Inc.
Recalls & Warnings
May 22, 2018
FDA Warns Companies Selling "Sun Protection" Supplements
On May 18, 2018, the FDA issued warning letters to four companies selling supplements for sun protection because statements made on product labels, websites and in marketing materials were found to be drug claims, which are not permitted for dietary supplements.
Recalls & Warnings
December 23, 2017
Seller of Meal Replacement, Protein Drinks, Cranberry and More Warned for Manufacturing Violations
On July 6, 2017, the FDA issued a warning letter to Professional Botanicals, Inc.
Recalls & Warnings
December 20, 2017
Seller of Supplements for Pain and Allergies Warned for Manufacturing Violations, Drug Claims
On December 13, 2017, the FDA issued a warning letter to GnuPharma Corporation, following a facility inspection which found a number of the company's products, including Relief capsules, Foundation capsules, Aller-geez capsules, Fit capsules, Aller-Geez Tea, Foundation herbal tea and ...
Recalls & Warnings
August 13, 2016
Seller of Omega-3, Ginkgo, Vision Supplements & More Warned for Drug Claims
On July 22, 2016, the FDA issued a warning letter to Viva Nutraceuticals (J & E International Corporation) following a review of the company's website and promotional literature, which found statement made about its products, Eyestonia, Ginkgo Biloba 60mg, Golden Omega-3 Max, ...
Recalls & Warnings
February 09, 2016
St. John's Wort Supplements Recalled
On February 8, 2016, the Medicines and Healthcare products Regulatory Agency (MHRA) (the British equivalent of the FDA) announced that certain St. John's wort supplements are being recalled because they contain excessive levels of a toxic pyrrolizidine alkaloid (PA).
Recalls & Warnings
June 22, 2010
Pet Vitamin Recalled for Salmonella Risk
On June 22, 2010, the FDA reported that United Pet Group, Cincinnati, Ohio is voluntarily recalling all unexpired lots of its PRO-PET ADULT DAILY VITAMIN Supplement tablets for Dogs due to possible Salmonella contamination. This product is not among those included in ConsumerLab.
Recalls & Warnings
June 09, 2020
Six More Multi-Level Marketing Companies Warned for Coronavirus and Deceptive Earnings Claims
On June 5, 2020, the FTC announced that it sent warning letters to six multi-level marketing companies for selling products such as immune system boosters and probiotics with unsupported claims that they can treat coronavirus (COVID-19) and/or for misrepresenting potential earnings people who have ...
Recalls & Warnings
June 04, 2020
FTC Warns 35 More Companies for Coronavirus Claims
On June 4, 2020, the FTC announced that it sent warning letters to 35 companies for selling products such as herbal products, immune system boosters, and vitamin C with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 15, 2020
FTC Warns 20 More Companies for Coronavirus Claims
On August 14, 2020, the FTC announced that it sent warning letters to 20 companies for selling products such as vitamin C, hydrochloroquine, omega 3, and melatonin with unsupported claims that they can treat coronavirus (COVID-19).
Recalls & Warnings
August 14, 2020
The Green Herb and New Genesis Health Warned for Manufacturing Violations
On July 31, 2019, the FDA issued a warning letter to Davis Ventures, Inc.
Recalls & Warnings
February 11, 2020
FTC Sues Two Companies Selling Bone Health and Joint Pain Supplements
On February 11, 2020, the Federal Trade Commission (FTC) announced that it sued two companies to stop them from continuing to make false claims about their bone and joint health products, Ostinol (ZyCal Bioceuticals) and StimTein (Excellent Marketing Results, Inc. (EMR)).
Recalls & Warnings
July 18, 2010
Expansion of Pet Supplement Recall for Salmonella Risk; Many Brands Affected
On July 2, 2010, the U.S.
Recalls & Warnings
November 11, 2013
Workout Supplement OxyElite Pro Recalled After Being Linked to Serious Liver Illness
On November 10, 2013, the FDA announced that USPLabs LLC is recalling workout supplements OxyElite Pro Super Thermo capsules, OxyElite Pro Ultra-Intense Thermo capsules and OxyElite Pro Super Thermo Powder because they have been linked to a number of cases of acute non-viral hepatitis, one of which ...
Recalls & Warnings
April 16, 2014
Seller of Aloe, Thyroid and Other Supplements Warned for Manufacturing Violations and Drug Claims
On March 21, 2014, the FDA issued a warning letter to Aloe Man International Corp.
Recalls & Warnings
March 31, 2005
FDA Warns Marketer of "Vitamin O" Product to Cease Unsubstantiated Claims
The U.S. Food and Drug Adminstration (FDA) has sent a Warning Letter (dated February 8, 2005) to Donald L. Smyth, President, R-Garden Inc., and Rose Creek Health Products, Inc. warning that its "Vitamin O" product was, among other things, being marketed with unsubstantiated health claims.
Recalls & Warnings
March 23, 2019
DG Health NATURALS Baby Cough Syrup Recalled
On March 20, 2019, Kingston Pharma, LLC issued a recall of all lots of DG/health NATURALS baby Cough Syrup + Mucus because it has the potential to be contaminated with Bacillus cereus/Bacillus circulans.
Recalls & Warnings
March 30, 2019
FDA Warns Seller of Reishi, Joint Supplements, Ginseng & More for Pesticides, Other Violations
On March 12, 2019, the FDA issued a warning letter to Yanqing "Michael" Li and Chang Su, owners of the website http://www.woohoonatural.
Recalls & Warnings
September 03, 2019
Federal Court Shuts Down Two Supplement Companies Selling Weight, Joint Health and Other Supplements
On September 3, 2019, a U.S. District Court entered a consent decree of permanent injunction against Basic Reset and Biogenyx, two Tennessee-based companies that sell dietary supplements and other product promoted for health benefits.
Recalls & Warnings
June 24, 2014
CELLFOOD Recalled in Canada
On June 23, 2014, Health Canada recalled CELLFOOD (Nu Science), a supplement promoted for improved energy, endurance and natural health, because it was found to be an unauthorized health product.
Recalls & Warnings
April 03, 2015
Health Canada Suspends Sales of Male Fern Products
On April 2, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced it has suspended the licenses of the natural health products containing the ingredient male fern (Dryopteris filix-max), due to concern over the safety of the ingredient when taken at higher doses.
Recalls & Warnings
August 31, 2006
Canada Advises Consumers Not To Use Adulterated Sleep Supplement
On August 30, 2006, Health Canada (the Candian Health Ministry) Health Canada advised consumers not to use Salt Spring Herbals Sleep Well Dietary Supplement because a sample analyzed by Health Canada has been found to contain the undeclared drug estazolam, a sedative that can be habit-forming when ...
Recalls & Warnings
January 10, 2018
Lithium Supplement Contaminated With E. Coli, Health Canada Warns
On December 1, 2018, Health Canada (the Canadian equivalent of the FDA) warned consumers that it found Smart Brain Formulations Serotonin Support, a lithium ororate supplement sold on the website Robert Lamberton Consulting, to be contaminated with E. choli (Escherichia coli).
Recalls & Warnings
July 06, 2012
Centrum Multivitamins: Breast and Colon Health Claims Pulled
On July 5, 2012, Pfizer Consumer Healthcare announced it will withdraw breast and colon health claims from its Centrum multivitamin advertising and labels, and will revise health and energy claims made on other Centrum products.
Recalls & Warnings
May 28, 2003
Warning and Recall for "Herbal" Sexual Enhancement Supplement in Canada Illegally Containing Pharmaceutical Compound
On May 27, 2003 - Health Canada warned consumers not to use Hua Fo VIGOR-MAX tablets, a Chinese herbal product that contains tadalafil.
Recalls & Warnings
March 19, 2007
Canada Warns of Weight Loss Supplement with Rx Drug
On March 14, 2007, Health Canada advised consumers not to use MIAOZI Slimming Capsules because they have been found to contain sibutramine, a prescription medication that should only be taken under medical supervision.
Recalls & Warnings
July 15, 2010
Supplement Company Pays $5.5 Million to Settle False Advertising Claims
On July 14, 2010, the Federal Trade Commission (FTC), as part of its ongoing efforts to stop bogus health claims, announced that it is requiring a major marketer of dietary supplements to pay $5.
Recalls & Warnings
December 26, 2010
Four Probiotic Products in Canada May Pose Serious Health Risks to Those with Milk or Soy Allergies
On December 24, 2010, Health Canada (the Canadian health ministry) advised consumers with milk or soy allergies that four probiotic natural health products are being voluntarily recalled from the market because they are labelled as not containing dairy (milk) and/or soy but may contain trace ...
Recalls & Warnings
June 07, 2012
Recall of Digestive Health Supplement Due to Salmonella Concern
On June 4, 2012, Botanical Laboratories of Ferndale, WA announced the recall of 38 bottles of 33.8 oz Wellese "Digestive 3 in 1 Health " liquid dietary supplement and 275 bottles of 16 oz.
Recalls & Warnings
July 28, 2015
Abnormal Heart Rhythms Linked with Unauthorized Natural Product, Health Canada Warns
On July 27, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced that it has received a serious adverse reaction report of abnormal heart rhythms associated with the use of an unauthorized natural product in Canada called Remogen, which contains the drug ibogaine.
Recalls & Warnings
April 06, 2018
Workout Supplements Recalled Due To Allergen Risk
On April 5, 2018, Independent Nutrition Inc, dba Back to Health of Eugene., issued a recall of certain lots of Ignite High Endurance Pre-Workout Supplements because they may contain undeclared milk.
Recalls & Warnings
March 09, 2016
Four Sexual Enhancement Supplements Recalled
On March 7, 2016, Health Canada (the Canadian equivalent of the FDA) announced that Vivo Brand Management is recalling four sexual enhancement products sold in Canada which may contain the undeclared drug sildenafil. Some of these products also appear to be available for purchase in the U.S.
Recalls & Warnings
March 02, 2016
Cyanide Danger With Certain Supplements, Health Canada Warns
On February 25, 2016, Health Canada (the Canadian equivalent of the FDA) warned that a number of products which were available for sale in Canada may contain a potentially toxic compound called amygdalin.
Recalls & Warnings
January 21, 2015
Supplement Maker Ordered to Stop Selling Products
On January 15, 2015, a federal judge ordered a permanent injunction against dietary supplement manufacturer Health One Pharmaceuticals, Inc. which requires the company to stop manufacturing and selling dietary supplements.
Recalls & Warnings
July 15, 2014
Vita Springs Health Warned for Manufacturing Violations and Drug Claims
On June 24, 2014, the FDA issued a warning letter to Albert Max Inc.
Recalls & Warnings
June 04, 2012
FDA Warning on Supplement for Pain Relief
On June 1, 2012, the U.S. FDA warned consumers that Reumofan Plus, marketed as a “natural” dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
Recalls & Warnings
November 26, 2012
Court Injunction Halts Sale of Cholesterol, CoQ10, Progesterone, Prostate Supplements and More
On November 20, 2012, Pharmacist's Ultimate Health agreed to cease sales and distribution of its dietary supplements by entering into a Consent Decree of Permanent Injunction in the U.S. District Court of Minnesota.
Recalls & Warnings
May 09, 2013
Joint Health Supplement Recalled for Undeclared Allergens
On May 1, 2013, XYMOGEN issued a voluntary recall of the joint health supplement artriphen, because it contains undeclared soy and milk allergens.
Recalls & Warnings
December 18, 2006
Canada Warns of Herbal Sleep Product Containing Rx Drug
On December 18, 2006 Health Canada advised consumers not to use a product called Eden Herbal Formulations Sleep Ease Dietary Supplement, because it was found to contain an undeclared drug estazolam, which can be habit-forming when used for as little as a few months.
Recalls & Warnings
April 09, 2003
Recall of Dangerous Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On April 4, 2003, the U.S. Food and Drug Administration's MedWatch program announced that Ultra Health Laboratories, Inc. and Bionate International, Inc. are warning consumers not to purchase or consume a product known as Vinarol tablets.
Recalls & Warnings
January 11, 2002
Canada Requests Recall of Certain Ephedra/Ephedrine Products
OTTAWA - January 10, 2002 - Health Canada is requesting a recall from the market of certain products containing Ephedra/ephedrine after a risk assessment concluded that these products pose a serious risk to health.
Recalls & Warnings
June 21, 2002
Canadian Warning on Use of Seven Herbal Supplements from BotanicLab
On June 19, 2002, Health Canada issued a warning that Canadians not use seven herbal products manufactured in the United States by BotanicLab because they contain undeclared prescription drugs that could cause serious health effects if not taken under medical supervision.
Recalls & Warnings
June 20, 2002
Canadian Warning on Use of Bejai Bowyantan for Infants
On June 14, 2002, Health Canada warned Canadians not to use Bejai Bowyantan for their young children and infants because it contains a substance with similar toxic properties to another substance known to cause serious adverse reactions and death in children.
Recalls & Warnings
March 11, 2002
Recall and Warning to Stop Use of Prostate Supplements "PC SPES" and "SPES"
Note: This posting was origially made on February 8, 2002. Additional information was added on March 11, 2002.
Recalls & Warnings
October 29, 2008
Two Vitamin C Supplements Recalled in Canada for Vitamin A Risk
On October 28, 2008, Health Canada warned Canadians, expecially expectant mothers, not to use two vitamin C products sold under the brand names New Roots Herbal Vitamin C8 and Vitazan Professional Vitamin C Advanced Ascorbate.
Recalls & Warnings
September 13, 2010
ExtenZe Enhancement Supplements Seized in Canada
On August 19, 2010, Health Canada (Canada's health ministry) seized the sexual enhancement supplements "Male Enhancement ExtenZe" and "Women ExtenZe" which were imported from the U.S. Although legal and widely sold in the U.S.
Recalls & Warnings
November 06, 2009
FDA Warns that "Stiff Nights" Enhancement Supplement Contains Undeclared Drug
On November 5, 2009 the U.S. Food and Drug Administration (FDA) warned consumers that Stiff Nights, a product marketed as a dietary supplement for sexual enhancement, contains an ingredient that can dangerously lower blood pressure and is illegal.
Recalls & Warnings
April 11, 2014
Digestive Health Supplement Recalled Due to Salmonella Risk
On April 4, 2014 Swanson Health Products issued a voluntary recall of digestive health supplement Swanson Premium Brand Full Spectrum Cilantro (Coriander), because it has the potential to be contaminated with Salmonella.
Recalls & Warnings
December 20, 2011
Organic Celery Seed Products Recalled Due to Potential Salmonella Contamination
On December 16, 2011, the U.S. FDA posted a notice from Swanson Health Products regarding the recall of Swanson Organic Celery Seed (Whole) which is packaged in plastic bottles with a net weight of 1.4 oz. (40 grams) because it has the potential to be contaminated with Salmonella.
Recalls & Warnings
August 06, 2015
Children and Adult Gummy Vitamins Recalled in Canada Due to Excessive Vitamin D
On August 6, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced Church & Dwight Canada Corp. is recalling the following supplements because they contain more vitamin D than claimed on the label (identified in Canada by their assigned natural product number (NPN):
Recalls & Warnings
November 28, 2015
Herbal Supplements Linked to Heavy Metal Poisoning
On November 17, 2015, Health Canada (the Canadian equivalent of the FDA) warned that a consumer in Canada who purchased and used herbal supplements from a U.S.-based website was treated for heavy metal poisoning.
Recalls & Warnings
April 24, 2018
Traditional Medicinals "Throat Coat" Lemon Echinacea Herbal Tea Recalled in Canada
On April 24, 2018, Health Canada (the Canadian equivalent of the FDA) advised consumers that one lot of Traditional Medicinals Throat Coat Lemon Echinacea herbal tea is being recalled in Canada because it has the potential to contaminated by Salmonella.
Recalls & Warnings
January 17, 2018
Joint and Pain Relief Product Contains Undeclared Drug
On January 17, 2018 Health Canada warned consumers that Linsen Double Caulis Plus, a supplement for joint pain, was found to contain the undeclared drug dexamethasone.
Recalls & Warnings
July 13, 2012
Alistrol Health Inc. Warned Over Health Claims on Products
On June 26, 2012, the FDA issued a warning letter to Alistrol Health Inc. for making product statements on the company's websites that constitute drug claims.
Recalls & Warnings
June 13, 2012
Tart Cherry Supplement Selller Warned by FDA Over Illegal Health Claims
The U.S. FDA recently published a Warning Letter (dated June 8, 2012) to Michelle's Miracle, Inc. for illegally promoting a range of tart cherry dietary supplements using health claims reserved for drugs. The products cited are:
Recalls & Warnings
October 05, 2007
FTC Charges Progesterone Cream Sellers with Making Unsubstantiated Claims
On October 5, 2007 the Federal Trade Commission (FTC) announced complaints against seven online sellers of alternative hormone replacement therapy (HRT) products, alleging that they made health claims for their natural progesterone creams without supporting scientific evidence.
Recalls & Warnings
December 22, 2008
FDA Warns Consumers About Tainted Weight Loss Pills
On December 22, 2008 the U.S. FDA alerted consumers not to purchase or consume any of more than 25 different products marketed for weight loss because they contain undeclared, active pharmaceutical ingredients that may put consumers’ health at risk.
Recalls & Warnings
January 19, 2002
Recall of Certain Iron Supplements
The U.S. Food and Drug Administration (FDA) released the following Class III recall information in its January 16, 2002 Enforcement Report. A Class III recall is a situation in which use of or exposure to a violative product is not likely to cause adverse health consequences:
Recalls & Warnings
October 06, 2005
Deceptive Marketing of “Supreme Greens" -- Settlement with FTC
On October 6, 2005, the Federal Trade Commission (FTC) announced that three individuals and two companies have settled FTC charges over their roles in the deceptive marketing of Supreme Greens, an herbal supplement.
Recalls & Warnings
June 26, 2006
FDA Warns Candy Maker Making Heart Health Claims
On May 31, 2006, the U.S.
Recalls & Warnings
March 03, 2006
FDA Requests Seizure of More Supplements Containing Ephedrine Alkaloids
On February 24, 2006, the U.S. Food and Drug Administration (FDA) announced that the U.S.
Recalls & Warnings
June 19, 2008
FDA Warns Groups to Stop Selling Fake Cancer 'Cures'
On June 17, 2008, the FDA reported that it had sent Warning Letters to 23 U.S. companies and two foreign individuals marketing a wide range of products fraudulently claiming to prevent and cure cancer.
Recalls & Warnings
April 11, 2008
Twelve Dietary Herbal Supplements Recalled -- Possible Health Risk Associated with Ephedra, Aristolochic Acid and Human Placenta
On April 10, 2008, the FDA posted a recall notice issued the same day from Herbal Science International, Inc. (AKA Jen-On Herbal Science International, Inc.).
Recalls & Warnings
April 07, 2008
Federal Agents Seize Nearly $1.3 Million of Illegal Dietary Supplements
On April 4, 2008 the U.S. FDA announced that, at its request, U.S. Marshals had seized more than $1,301,712 of dietary supplements from LG Sciences, LLC, of Brighton, Mich.
Recalls & Warnings
March 28, 2008
FDA Warns of Adverse Reactions with Two Liquid Supplements -- Selenium Toxicity Possible
The U.S. Food and Drug Administration is advising consumers not to purchase or consume Total Body Formula in the flavors of Tropical Orange and Peach Nectar, or Total Body Mega Formula in the Orange/Tangerine flavor.
Recalls & Warnings
January 31, 2010
Glucomannan May Cause Choking if Not Taken with Fluid
On January 29, 2010, the Canadian health department, Health Canada, adised that natural health products containing the ingredient glucomannan in tablet, capsule or powder form have a potential for harm if taken without at least 8 ounces of water or other fluid.
Recalls & Warnings
December 08, 2009
Warning on Acai Berry Supplements Spiked with Drug
On December 8, 2009, Health Canada (the Canadian health agency) advised consumers not to use certain Acai Berry products after a large number of shipments of adulterated products were stopped at the border.
Recalls & Warnings
May 03, 2011
FDA Warns: Beware of Bogus STD Products
On May 3, 2011, the U.S. Food and Drug Administration (FDA) and the Federal Trade Commission (FTC) announced a joint effort to remove products from the market that make unproven claims to treat, cure, and prevent sexually transmitted diseases (STDs).
Recalls & Warnings
July 03, 2012
Standard Process Recalls Three Supplements Due to Possible Salmonella Contamination
On June 29, 2012, Standard Process issued a voluntary recall of the company's Cataplex ACP, Cataplex C and Pancreatrophin PMG, after a routine FDA inspection uncovered potential Salmonella contamination in an ingredient found in these three products.
Recalls & Warnings
November 01, 2013
Protein Shakes Containing Prescription Drug Pose Serious Health Risk
On November 1, 2013, Health Canada warned consumers that Vega One Vanilla Chai (sizes 437g and 874g) and Vega Sport Performance Chocolate nutritional shakes, by Sequel Naturals Ltd., have been found to contain the prescription antibiotic chloramphenicol.
Recalls & Warnings
October 27, 2015
Serious Cardiovascular Risks Associated with Strontium, Health Canada Warns
Strontium supplements should not be taken by people with a history of, or risk factors for heart disease, circulatory problems, or blood clots, according to a safety review conducted by Health Canada (the Canadian equivalent of the FDA).
Recalls & Warnings
April 17, 2015
Herbal Laxative and "Detox" Kit Containing High Levels of Lead and/or Arsenic Recalled in Canada
On April 15, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced that St. Francis Herb Farm Inc.
Recalls & Warnings
June 17, 2015
Maker of Joint Supplement Warned for Manufacturing Violations
On May 29, 2015, the FDA issued a warning letter to Total Health Advanced Nutrition, Inc.
Recalls & Warnings
February 06, 2015
POM Wonderful Claims Were Deceptive, Court Rules
On January 30, 2015, the Federal Trade Commission (FTC) announced that an appeals court has upheld the Commission's ruling that marketers for POM Wonderful made deceptive health claims about the product.
Recalls & Warnings
July 26, 2014
Marketers of Nopal Cactus Drink Settle FTC Charges of Deceptive Claims
TriVita Inc., and marketers of the company's "prickly pear" fruit drink Nopalea have agreed to pay $3.5 million in consumer refunds to settle FTC charges they made deceptive claims that the drink treats health problems ranging from skin conditions to joint pain and respiratory problems.
Recalls & Warnings
November 06, 2014
Seller of Prostate, Heart Supplements and More Warned for Drug Claims
On October 9, 2014, the FDA issued a warning letter to Health Research Laboratories, LLC/New World Health, following a review of the company's websites, which found statements made about AtheChel Advanced, Betarol, BioTherapex, Omega-3 Cardio Plus, RejuvaLifeRx, and Ultimate Health Formula to be ...
Recalls & Warnings
October 31, 2014
Seller of "Cleanse," Allergy Supplements and More Warned for Manufacturing Violations, Drug Claims
On October 15, 2014, the FDA issued a warning letter to Evangelical Christian Ministries, dba Health & Herbs, following a facility inspection which found the company's products, CORYDALIS and CVF BGONE to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
December 12, 2014
Seller of B-12, Zinc, Echinacea and More Warned for Manufacturing Violations and Drug Claims
On November 14, 2014, the FDA issued a warning letter to Scientific Botanicals Company, Inc.
Recalls & Warnings
December 01, 2017
Health Research Labs Agrees to Settle FTC Charges of False Claims, Deceptive Marketing of BioTherapex and NeuroPlus
On November 30, 2017, the FTC announced Health Research Laboratories LLC and owner, Kramer Duhon, agreed to settle charges that they made false claims about the company's products, BioTherapex and NeuroPlus.
Recalls & Warnings
February 01, 2018
Seller of Supplements for Opiate Withdrawal Warned for Drug Claims
On January 11, 2018, the FDA sent warning letters to ten sellers of supplements promoted to treat opiate withdrawal following reviews of the companies' websites and social media which found statements and testimonials made about the products to be drug claims.
Recalls & Warnings
July 31, 2018
FDA Warns Seller of Joint Health and Cholesterol Supplements
On July 13, 2018, the FDA issued a warning letter to GC Natural, following a facility inspection which found a number of the company's products, including Red Pyrola Plus + Advanced Joint Formula Capsules, CSDP GOLD Capsules, Rejeune Optimal Kidney & Liver Support Extract balls, C.
Recalls & Warnings
April 01, 2017
Maker of Prelief, Urinozinc Prostate Health Formula Warned for Manufacturing Violations
On March 16, 2017 the FDA issued a warning letter to DSE Healthcare Solutions, LLC, following a facility inspection which found the company's products, including Prelief and Urinozinc Prostate Health Formula to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
February 28, 2017
Multivitamin Recalled Due to Allergen Risk
On February 14, 2017, Licata Enterprises issued a recall of all lots of its Supreme One and Theravits 100 multiple vitamins because they contain fish liver oil but is labeled as containing "no common allergens.
Recalls & Warnings
March 12, 2016
FDA Finds Problems at 58% of Supplement Manufacturing Sites in U.S. and Abroad
ConsumerLab.com has obtained results of the FDA's inspections in Fiscal Year 2015 (ending September 30) of 483 dietary supplement manufacturing facilities, showing that most -- 58.2% -- received letters indicating noncompliance with current Good Manufacturing Practices (cGMPs).
Recalls & Warnings
April 08, 2003
FDA Acts Against Potentially Risky Products Illegally Marketed as Street Drug Alternatives
On March 31, the Food and Drug Administration (FDA) announced enforcement actions against firms that are marketing street drug alternative products, some of which contain ephedra or other sources of ephedrine.
Recalls & Warnings
January 27, 2003
FTC Charges Dr. Clark and Related Companies with Making Unsubstantiated Health Claims
The Federal Trade Commission has charged a Switzerland-based company and its U.S. counterpart with making numerous unsubstantiated efficacy claims for a variety of dietary supplements and devices that they sell on the Internet.
Recalls & Warnings
January 03, 2004
FDA to Prohibit Sales of Dietary Supplements Containing Ephedra
On December 30, 2003, the U.S. Food and Drug Administration (FDA) issued a consumer alert of its forthcoming determination that dietary supplements containing ephedra present an unreasonable risk of illness or injury, and should not be consumed.
Recalls & Warnings
March 25, 2008
FDA Warns of Sexual Supplements with Drug-like Ingredients
On March 25, 2008, the U.S. Food and Drug Administration advised consumers not to purchase or use "Blue Steel" or "Hero" products marketed as dietary supplements throughout the United States because they are considered unapproved drugs and have not been proven to be safe or effective.
Recalls & Warnings
May 10, 2007
FDA Warns of Two Supplements Containing Pharmaceutical-like Compounds
On May 10, 2007, the FDA advised consumers not to purchase or use "True Man" or "Energy Max" products promoted and sold as dietary supplements throughout the United States.
Recalls & Warnings
October 12, 2005
Supplement Company to Pay $2 Million Penalty For Alleged Violations of FTC Order
On October 12, 2005 the Federal Trade Commission (FTC) announced that under the terms of a consent decree approved by the it for submission by the U.S. Department of Justice (DOJ) to the federal court for approval, NBTY, Inc. (NBTY, formerly Nature’s Bounty, Inc.
Recalls & Warnings
October 06, 2006
FDA Warns Mangosteen Juice Maker of Drug Promotions
On September 20, 2006, the U.S. Food and Drug Administration (FDA) sent a Warning Letter to the makers of Xango, a mangosteen juice product. The letter advised that distributors of the product were using brochures making health claims restricted to the promotion of drugs.
Recalls & Warnings
January 09, 2009
FDA Expands Warning to Consumers About Tainted Weight Loss Pills
On January 8, 2009, the U.S. FDA expanded its nationwide alert to consumers about tainted weight loss pills that contain undeclared, active pharmaceutical ingredients. On December 22, 2008, FDA warned consumers not to purchase or consume 28 different products marketed for weight loss.
Recalls & Warnings
May 03, 2010
FDA Warns Consumers to Avoid Breath Supplement Contaminated with Lead
On May 3, 2010 the U.S. Food and Drug Administration warned consumers not to purchase nor consume Vita Breath, a dietary supplement manufactured by American Herbal Lab Inc. of Rosemead, Calif.
Recalls & Warnings
August 25, 2009
Hepatitis Associated with Herbal Supplement Containing Artemisinin
The August 14, 2009 edition of the Center for Disease Control's (CDC) Morbidity and Mortality Weekly Report includes case report of hepatitis associated with use of an herbal supplement containing artemisinin.
Recalls & Warnings
August 09, 2010
FDA Warns Against "Miracle" Mineral Supplement
On July 30, 2010, The U.S. FDA warned consumers not to consume or use Miracle Mineral Solution, an oral liquid solution also known as "Miracle Mineral Supplement" or "MMS." The product, when used as directed, produces an industrial bleach that can cause serious harm to health.
Recalls & Warnings
September 27, 2010
FTC Charges Deceptive Advertising by POM Wonderful
On September 27, 2010, the U.S.
Recalls & Warnings
July 20, 2010
Recall of ED Supplement Containing Drug
On July 20, 2010, the U.S. FDA posted a news announcement from Good Health, Inc. regarding the recall of Vialipro, a sexual enhancement supplement sold nationally.
Recalls & Warnings
November 18, 2003
Supplement Promoted As Cancer Treatment Removed from Market
On November 10, 2003, the Food and Drug Administration (FDA) announced that NBTY, Inc., of Bohemia, N.Y.
Recalls & Warnings
December 11, 2002
Recall of Calcium Supplement Possibly Contaminated with Antibiotic
The U.S. Food and Drug Administration (FDA) released the following Class II recall information in its December 11, 2002 Enforcement Report.
Recalls & Warnings
September 16, 2005
FTC Stops Weight-loss Claims about Seaweed-based Patches
On September 15, 2005, the U.S. Federal Trade Commission (FTC) announced that the alleged masterminds behind a fraudulent scheme to market two seaweed-based patches as weight-loss products to U.S. consumers have settled FTC charges.
Recalls & Warnings
November 03, 2004
FDA Warns of Sexual Enhancement Supplements Containing Prescription Drug
On November 2, 2004, the Food and Drug Administration (FDA)warned consumers not to purchase or to consume Actra-Rx or Yilishen, two products promoted and offered for sale on Web sites as "dietary supplements" for treating erectile dysfunction and enhancing sexual performance for men.
Recalls & Warnings
August 31, 2004
Two Makers of Weight Loss and Sex Enhancement Supplements Stopped From Making Unsubstantiated Claims
On August 27, 2004, the Federal Trade Commmission (FTC) reported that two Maine-based dietary supplement marketers and their principals have agreed to settle FTC charges that they made deceptive advertising claims about their dietary supplement products, in violation of federal law.
Recalls & Warnings
May 03, 2002
More Information on Recall of PC-SPES - Prostate Supplement
The FDA's May 1, 2002 Enforcement Report classified the recall of PC-SPES as a Class I recall. The recall was originally announced by its manufacturer in February with additional warnings from the FDA issued in March (see the 3/11/02 posting on ConsumerLab.
Recalls & Warnings
August 09, 2002
Recall of 7 Herbal Products from Botanic Lab in US and Abroad
The U.S. Food and Drug Administration (FDA) released the following Class II recall information in its August 7, 2002 Enforcement Report.
Recalls & Warnings
September 25, 2018
Seller of B Vitamins, Multis, Glucosamine & More Warned for Manufacturing Violations
On August 31, 2018, the FDA issued a warning letter to Independent Nutrition Inc., following a facility inspection which the company's products, including B-50 Complete, Multi-Vitamin & Mineral Complex (a.k.a.
Recalls & Warnings
September 25, 2018
FDA Warns Seller of Digestive Enzyme Supplements
On March 6, 2018, the FDA issued a warning letter to Uckele Health & Nutrition, Inc.
Recalls & Warnings
September 19, 2018
Seller of Curcumin, Garcinia, Glucosamine and More Warned for Manufacturing Violations
On September 7, 2018, the FDA issued a warning letter to Best Nutrition Products, Inc.
Recalls & Warnings
January 30, 2018
Limbrel for Osteoarthritis Recalled Due to Risk of Adverse Effects
On January 26, 2018, Primus Pharmaceuticals, Inc. of Scottsdale, Arizona issued a recall of all unexpired lots of Limbrel products.
Recalls & Warnings
January 21, 2017
Seller of "Cancer Kits," Alpha Lipoic Acid Supplements and More Warned for Drug Claims
On December 22, 2016, the FDA issued a warning letter to Northern Health Products, Inc. following a review of the company's websites, www.northernhealthproducts.com and www.petdca.
Recalls & Warnings
August 31, 2016
Seller of Joint Supplement Warned for Manufacturing Violations, Drug Claims
On July 15, 2016, the FDA issued a warning letter to Vitalife Inc.
Recalls & Warnings
August 29, 2017
FDA Warns Seller of Supplements for Allergies, Joint Pain, Bone Health, and More
On August 16, 2017, the FDA issued a warning letter to Total Nutrition, Inc.
Recalls & Warnings
May 30, 2017
Maker of Collagen Supplement Warned for Manufacturing Violations
On July 17, 2014, the FDA issued a warning letter to Morhaim Pharmalab, Inc.
Recalls & Warnings
December 29, 2015
Maker of Vitamin K, Vitamin A & More Warned for Manufacturing Violations, Drug Claims
On December 10 2015, the FDA issued a warning letter to Dherbs Health Emporium, Inc.
Recalls & Warnings
March 22, 2016
NOW Foods Recalls Ginkgo & Other Supplements
On March 18, 2016, NOW Foods issued a recall of the following dietary supplements due to errors on the labels:
Recalls & Warnings
July 02, 2015
Maker of Arthritis Supplement Warned for Manufacturing Violations, Drug Claims
On June 17, 2015, the FDA issued a warning letter to Desert Stream, Inc.
Recalls & Warnings
October 21, 2015
Imported Supplements Can Be Dangerous, FDA Warns
On October 15, 2015, the FDA warned consumers that some supplements sold at nontraditional places, such as ethnic or international stores, flea markets, swap meets, as well as online, are often imported from other countries, and some can potentially be harmful.
Recalls & Warnings
August 19, 2015
Soylent Meal Replacement Powder Reported to Contain Lead and Cadmium
On August 13, 2015, an environmental-health watchdog group, As You Sow, filed a notice of intent to bring legal action against Soylent, a "meal replacement" powder, alleging violation of California's "Prop 65" Safe Drinking Water and Toxic Enforcement Act ...
Recalls & Warnings
January 31, 2015
Maker of Soy and Zinc Warned for Manufacturing Violations
On December 18, 2014, the FDA issued a warning letter to PreMark Health Science, Inc.
Recalls & Warnings
January 27, 2015
Green Coffee Bean Supplement Marketer Settles FTC Charges of Deceptive Claims
On January 26, 2015, the FTC announced that Pure Health LLC, Genesis Today, and owner of both companies, Lindsey Duncan, will pay $9 million to settle charges of deceptive weight loss claims made about the companies' green coffee bean extract products.
Recalls & Warnings
May 30, 2015
Seller of Supplements for Herpes, Prostate Cancer & More Warned for Drug Claims
On May 7, 2015, the FDA issued a warning letter to Strictly Health Corporation, following a review of the company's websites which found statements made about FENVIR, Prosta Pep and Tonalin brand CLA to be drug claims.
Recalls & Warnings
May 02, 2015
Maker of Garcinia, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On March 25, 2015, the FDA issued a warning letter to JW Nutritional LLC, following a facility inspection which found the company's products, including Vanish V2 Domestic, Mr.
Recalls & Warnings
December 05, 2014
Maker of Ginseng, Zinc, Calcium and More Warned for Manufacturing Violations
On November 20, 2014, the FDA issued a warning letter to Long Island Pharmaceuticals, LLC, following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing Practices for dietary ...
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
September 10, 2014
Seller of Joint Pain Supplement Warned for Drug Claims
On August 24, 2014, the FDA issued a warning letter to CreAgri, Inc., following a website review, which found statements made about the company's products, including Olivenol plus Easeflex, Olivenol plus Essence Capsules, and Olivenol plus Essence Elixer, to be drug claims.
Recalls & Warnings
August 26, 2014
Supplement Maker Warned for Manufacturing Violations
On August 14, 2014, the FDA issued a warning letter to dietary supplement manufacturer Big Easy Confections following a facility inspection which found the company's products to be adulterated because they were prepared, packed, or held under conditions that violate Current Good Manufacturing ...
Recalls & Warnings
August 21, 2014
Seller of Joint Supplement Warned for Manufacturing Violations and Drug Claims
On July 17, 2014, the FDA issued a warning letter to Klein Laboratories, Inc.
Recalls & Warnings
August 06, 2014
Seller of Mineral and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On July 25, 2014, the FDA issued a warning letter to Mezotrace Corporation following a facility inspection which found the company's products, including Calcium/Magnesium Natural Minerals & Trace Elements, Calcium/Magnesium Natural Minerals & Trace Elements with Vitamin D, and Calcium/Magnesium ...
Recalls & Warnings
August 04, 2014
"Superfood" Greens Recalled Due to Salmonella Risk
On August 1, 2014, New England Greens dba Vibrant Health issued a recall of 10 lots of Green Vibrance and one lot of Rainbow Vibrance because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
October 09, 2013
Use of Chinese Supplement Linked to Rare But Serious Condition
On October 9, 2013, Health Canada warned consumers that Compound Danshen Dripping Pills, manufactured by Tianjin Tasly Pharmaceutical Co., have been associated with a case of methemoglobinemia, a rare but serious condition which may result in coma or death.
Recalls & Warnings
January 16, 2014
Joint Health Supplement Contains Dangerous Drugs, FDA Warns
On January 15, 2014, the FDA warned consumers not to buy or use joint health supplement Pro ArthMax (by Human Science Foundation) because it was found to contain multiple drugs, including diclofenac, ibuprofen, naproxen, indomethacin, nefopam, and chlorzoxazone.
Recalls & Warnings
December 19, 2013
Maker of Joint Health Supplements Warned for Manufacturing Violations
On December 4, 2013, the FDA issued a warning letter to PurQuality, LLC.
Recalls & Warnings
May 10, 2013
Protein Extract Recalled for Undeclared Allergens
On May 6, 2013, Pure Herbs Ltd. issued a voluntary recall of Protein Extract because it contains undeclared milk and soy allergens.
Recalls & Warnings
June 05, 2014
More Chia Powders Recalled Due to Salmonella Risk
On June 4, 2014, Health Matters America Inc. issued a voluntary recall its Organic Traditions Sprouted Chia Seed Powder and Organic Traditions Sprouted Chia & Flax Seed Powder because they have the potential to be contaminated with Salmonella.
Recalls & Warnings
September 17, 2013
Seller of Omega-3 and Joint Supplements Warned for Manufacturing Violations and Drug Claims
On August 29, 2013, the FDA issued a warning letter to Y.S. Health Corp.
Recalls & Warnings
April 07, 2011
Prostate Drug Found in Prostate Supplement -- Recall Underway
On March 24, 2011 the U.S. FDA posted a voluntary recall notice for U-Prosta from USA Far Ocean Group, Inc.
Recalls & Warnings
September 10, 2012
Maker of Omega-3, Bone and Brain and Supplements Warned For Drug Claims
On August 27, 2012, the FDA issued a warning letter to PruTect Rx indicating that statements on the company's websites about Omega3PruTect, NeuroPruTect, VitaminD3PruTect and OsteoPruTect constitute drug claims, although the products are not approved drugs.
Recalls & Warnings
August 31, 2012
Recent Cases of Lead Poisoning From Ayurvedic Medications from India
Public health officials have reported six cases of lead poisoning among foreign-born women who took Ayurvedic herbal products imported from India. The cases were reported by the New York City Department of Health and Mental Hygiene.
Recalls & Warnings
November 28, 2012
Maker of Pycnogenol, Memory and Immune Supplements Warned For Manufacturing Violations and Misbranding
On November 15, 2012, the FDA issued a warning letter to contract manufacturer Health Technology, Inc.