Showing Results for Muscle Enhancement
Search term may appear only in full report available to members. Join now for full access.
Product Review
Muscle & Workout Supplements Review (Creatine and Branched-chain Amino Acids)
Do Creatine and BCAAs Really Improve Strength and Recovery?

News Release
June 08, 2023
Best Muscle and Workout Supplements With Creatine or BCAAs, According to ConsumerLab Tests
White Plains, New York, June 8, 2023 — Creatine supplements are promoted to increase muscle size, strength, and endurance, while supplements with branched-chain amino acids (BCAAs) are promoted to improve exercise recovery.
News Release
June 30, 2022
Best Protein Shakes & Drinks According to ConsumerLab Tests. Lead, Excess Sodium Found in Some
White Plains, New York, June 30, 2022 — Consumers may get more sodium, fat, calories, or even lead, than they bargained for from some protein supplements, according to recent ConsumerLab tests of over 20 popular protein powders and shakes.
CL Answer
Is there a risk of liver toxicity with certain supplements?
Find out if there is risk of liver damage from supplements such as green tea, niacin, red yeast rice and vitamin A.
CL Answer
What is BPC-157, what is it used for and is it safe?
Find out about BPC-157, including what it's promoted for and if it is safe.
News Release
July 01, 2015
Tests of Maca "Enhancement" Supplements Find Real Maca, But Some Lead Contamination and Seemingly Excessive Amounts of Rice Filler
White Plains, New York, July 1, 2015 — It's touted for increasing stamina and sexual performance and sales have steadily increased for more than a decade, but do maca supplements really contain this root indigenous to Peru? To answer this question, ConsumerLab.
Product Review
Breast Enhancement Supplements Review Article
Do Breast Enhancement Supplements Really Work? See the Evidence For and Against Breast Enhancement Supplements.

News Release
January 15, 2014
Problems Found with the Quality and Labeling of Some "Muscle Enhancement" Supplements -- Review of Creatine and Branched-chain Amino Acid Supplements Published by ConsumerLab.com
White Plains, New York, January 15, 2014 — Athletes often turn to supplements such as creatine and branched-chain amino acids (BCAAs) to enhance muscle strength and recovery. These supplements may also benefit people with muscular diseases and those recovering from knee surgery.
Product Review
Nitric Oxide Supplements (Bodybuilding and Athletic Performance) Review Article
Do Nitric Oxide Supplements Really Work? Find Out If Nitric Oxide Supplements Really Build Muscle or Improve Performance.

News Release
February 05, 2012
Fish oil and multivitamins most popular supplements in ConsumerLab.com survey -- Internet most popular place to shop
WHITE PLAINS, NEW YORK — FEBRUARY 5, 2012 — A survey of over 10,000 savvy consumers of supplements shows the most popular supplements to be fish oil, multivitamins, vitamin D, calcium and CoQ10, in that order.
CL Answer
Is it safe to take arginine, or other supplements for sexual enhancement, while taking Viagra or Cialis?
When taking Cialis or Viagra, is it safe to take arginine or other sexual enhancement supplements?

CL Answer
Can taurine help reduce muscle cramps?
Find out if taurine supplements can help reduce muscle cramps in people with liver damage (cirrhosis), reduce muscle soreness after exercise, or have other benefits. ConsumerLab.com's answer explains.
CL Answer
Does urolithin A reduce age-related muscle decline or improve osteoarthritis?
Find out if urolithin A may improve age-related muscle decline and learn about its safety.

CL Answer
Do essential amino acid (EAA) supplements help build muscle better than dietary protein or protein supplements?
Comparison of essential amino acids vs dietary protein and protein supplements for building muscle.

CL Answer
Does taking creatine affect tests for creatinine in urine samples or creatine kinase in blood samples?
Find out if creatine can affect creatinine levels and kidney function and whether creatine affects creatine kinase activity, a measure of muscle injury.

News Release
November 14, 2011
ConsumerLab.com examines whether nitric oxide supplements make muscles bigger and increase endurance
White Plains, New York — November 14, 2011 — Nitric oxide supplements are widely used for bodybuilding and athletic performance, but do they really work? "They won't pump up your muscles — their main claim, but ‘nitric oxide' supplements may help you exercise intensely a little ...
CL Answer
Supplements for Age-Related Muscle Loss
Find out if protein supplements, fish oil, vitamin D, and other supplements can help older adults build and maintain muscle, and help prevent age-related muscle loss (sarcopenia).

News Release
August 31, 2011
ConsumerLab.com analyzes supplements for sexual dysfunction -- Only three out of ten products selected for testing pass
WHITE PLAINS, NEW YORK — August 31, 2011 — Only three out of ten supplements selected for testing that are for sexual enhancement passed ConsumerLab.com's latest investigation.
CL Answer
I do moderate exercise for about an hour a few times a week. Which supplements might help me, and which should I consider avoiding?
Info on what supplements are best for work out & exercise regimens, including electrolytes, protein, creatine, and BCAAs.

CL Answer
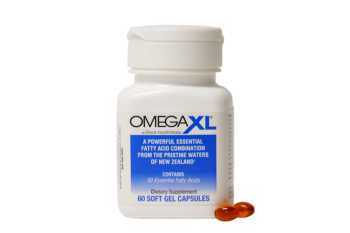
What is Omega XL and does it work?
What is Omega XL (distributed by Great Health Works) and does it work? Described as a "highly concentrated Omega-3 'Super Oil' supplement".
CL Answer
Does forskolin help for weight loss? Is it safe?
Learn more about forskolin including weight loss, diet, preventing asthma attacks and its proposed effects on the body.
CL Answer
Do any supplements, foods, or oils help reduce muscle pain, muscle cramps, or nighttime leg cramps?
Find out which supplements, and topical products such as magnesium creams, can help reduce muscle pain, leg cramps, and nighttime leg cramps, and which may worsen muscle cramps. Also, learn about vitamin and mineral deficiencies and common medications that can cause muscle cramps and nocturnal leg cramps.
CL Answer
Is Greek yogurt a good source of protein?
Find out if Greek yogurt is a good source of protein, how much protein it contains, and what type of protein it contains..

CL Answer
Does D-aspartic acid boost testosterone levels?
Find out if D-aspartic acid (aspartic acid) raises testosterone levels, increases libido or muscle mass.

Product Review
L-Arginine Supplements Review
Choose the Best L-Arginine Supplement. Find Out Which L-Arginine Supplement Passed CL's Tests.

CL Answer
Which supplements help with erectile dysfunction?
Supplements for erectile dysfunction. Find out which supplements can help with ED, including ginseng, L-arginine, and maca.

Product Review
CoQ10 and Ubiquinol Supplements Review
Find the Best CoQ10 and Ubiquinol Supplements and Learn How They Differ

CL Answer
Taking 2 grams of L-glutamine in water on an empty stomach (as directed) caused me to be nauseous, dizzy and anxious. Is this a normal side effect of glutamine?
Find out how to prevent nausea, dizziness and anxiety when taking monosodium glutamine in water on an empty stomach.

CL Answer
Are there supplements I should take, or take differently, when intermittent fasting?
Supplements that may help when intermittent fasting. Information about whey protein, CoQ10, magnesium and others, plus coffee, fiber and more.

CL Answer
What are the symptoms of vitamin D deficiency?
Learn about the symptoms of vitamin D deficiency, such as soft or weak bones, bone, joint and muscle pain, depression, insomnia, hair loss and others, and find out how much vitamin D you need. ConsumerLab.com's answer explains.

Recalls & Warnings
June 20, 2017
Beware of Bodybuilding Supplements, FDA Warns
On June 20, 2017, the FDA warned consumers to beware of bodybuilding products because a number of these products have been found to contain steroids or steroid-like substances. Such products could cause serious and even life-threatening health risks, including:
CL Answer
Is bromelain a good alternative to NSAIDs, like ibuprofen, for muscle or joint pain?
Do bromelain supplements have benefits? Learn more about its safety, and if it is a good alternative to NSAIDs like ibuprofen.

CL Answer
I have heard that caffeine can interfere with the benefits of creatine supplementation. Is that true?
Find out if creatine supplements are impacted by caffeine intake.
CL Answer
Does citrulline improve exercise performance or recovery?
Information on citrulline supplements helping with exercise endurance, performance, muscle strength, fatigue or soreness after exercise. ConsumerLab.com's answer explains.

CL Answer
What is the best protein supplement for vegetarians and vegans?
Find out which protein supplements for vegetarians and vegans are best, including those made from whey, casein, soy, rice, pea, and hemp. ConsumerLab.com's answer explains.

CL Answer
Are L-carnosine supplements helpful and safe?
Learn about the potential health benefits of L-carnosine, as well as possible safety concerns, drug interactions and cost.

CL Answer
10 Supplements That May Boost Testosterone (And 12 That May Not)
Many supplements promise to increase testestorone, boost libido, and increase muscle size, but, as ConsumerLab explains, the actual effectiveness of supplements is limited, and some supplements may actually reduce testosterone levels.
CL Answer
Is topical menthol, such as Biofreeze, safe and effective for pain relief?
Menthol is commonly used in topical creams and gels for pain relief. Find out if it works and if it's safe.
CL Answer
What is GABA (gamma-aminobutyric acid)? Is it beneficial for stress, anxiety, insomnia or other conditions, and is it safe?
Find out about GABA (gamma-aminobutyric acid), including if it's beneficial for insomnia, epilepsy, stress and anxiety, high blood pressure and building muscle, and learn if taking GABA supplements is safe.

Recalls & Warnings
May 20, 2017
Muscle Enhancement Supplement Containing Steroid-Like Substances Recalled
On May 19, 2017, Dynamic Technical Formulations LLC issued a recall of all lots its muscle enhancement supplement Tri-Ton because it was found to contain anabolic steroid-like substances (andarine and ostarine) which are not permitted in dietary supplements.
Recalls & Warnings
July 10, 2017
Bodybuilding Supplements Containing Steroid-Like Substances Recalled
On June 29, 2017, Hardcore Formulations recalled all lots and expiration dates of their products Ultra-Sten and D-Zine, bodybuilding supplements found to contain methylstenbolone and dymethazine, which are derivatives of anabolic steroids.
News Release
July 21, 2010
Tests of "muscle enhancement" supplements show quality problems with some creatine and branched-chain amino acid products -- Review of Muscle Enhancement Supplements published by ConsumerLab.com
WHITE PLAINS, NEW YORK — July 21, 2010 — Athletes often turn to supplements to enhance muscle strength and recovery. New tests of such supplements by ConsumerLab.
News Release
November 18, 2008
Adulteration Suspected with Some "Memory" Supplements -- Few Ginkgo and Huperzine Supplements Pass ConsumerLab.com Tests; Quality High for Acetyl-L-Carnitine
WHITE PLAINS, NEW YORK — NOVEMBER 18, 2008 — Tests by ConsumerLab.com of Ginkgo biloba supplements show that few products meet quality standards.
News Release
November 17, 2006
ConsumerLab.com responds to vitamin industry claim to be "vindicated" in defamation case
WESTCHESTER, NEW YORK — November 17, 2006 — ConsumerLab.com (www.consumerlab.com), an independent evaluator of health and nutrition products, responded to a press release issued today by the vitamin lobbyist/trade group called the Council for Responsible Nutrition (CRN).
Recalls & Warnings
July 12, 2017
More Bodybuilding Supplements Recalled
On July 11, 2017, Andropharm recalled two bodybuilding supplements, Sten Z and M1 Alpha, because these products contain derivatives of anabolic steroids.
News Release
November 13, 2006
Tests of "muscle" supplements finds some "weak" products but most contain expected creatine, HMB, or amino acids — Review of muscular enhancement supplements published by ConsumerLab.com
WESTCHESTER, NEW YORK — MONDAY, NOVEMBER 13, 2006 — Bodybuilders and athletes often turn to supplements to enhance muscle size and strength.
News Release
October 20, 2006
ConsumerLab.com report on CoQ10 supplements finds quality high but large range in suggested dosage
WHITE PLAINS, NEW YORK — OCTOBER 20, 2006 — New test results from ConsumerLab.com for CoQ10 supplements show that all of the products selected contained amounts of ingredient consistent with their labels.
News Release
October 10, 2006
Care advised with natural cold remedies: ConsumerLab.com finds few products with proper quality and directions — ConsumerLab.com offers advice from tests of echinacea, garlic, ginseng, vitamin C, and zinc supplements
WHITE PLAINS, NEW YORK — TUESDAY, OCTOBER 10, 2006 — Autumn is the beginning of "cold season" — the period through early spring when people are most likely to develop a cold. But based on ConsumerLab.
Clinical Update
12/07/2019
Vitamin D & Muscle
If you are looking to increase muscle mass, make sure you're getting adequate vitamin D. Raising deficient levels has been shown to increase muscle in seniors, and more recently, in men and women in their 40s. For details see the Muscle, balance and falls section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
12/09/2017
Creatine Pumps Up Muscles
Giving creatine during a resistance-training program significantly boosted muscle size more than placebo in a recent study — and certain muscles increased in size more than others. Learn more in the "What It Does" section of the Muscle & Workout Supplements Review (Creatine & BCAAs). (Also see our Top Picks and Cautions and Concerns for creatine).
Clinical Update
9/15/2020
Preventing Muscle Loss with Leucine?
Do supplements with leucine (a branched-chain amino acid or BCAA) prevent muscle loss when muscles are immobilized (such as in a leg brace)? See what a recent study showed in the What It Does section of our Muscle & Workout Supplements Review.
Clinical Update
1/27/2021
Curcumin for Reducing Muscle Soreness?
Can taking curcumin (from turmeric) reduce muscle soreness related to exercise – and does it enhance muscle strengthening from exercise? See what a new study found in the Muscle Soreness and Recovery section of the Turmeric and Curcumin Supplements & Spices Review. Also see our Top Picks for turmeric/curcumin.
Recalls & Warnings
December 26, 2023
Total Body Nutrition, TBN Labs, and Loud Muscle Science Banned from Selling Adulterated and Misbranded Dietary Supplements
On December 11, 2023, Total Body Nutrition LLC, TBN Labs LLC, and Loud Muscle Science LLC, as well as the companies’ owner, Mohammed Islam, were prohibited by federal court from manufacturing and distributing adulterated and misbranded dietary supplements.
News Release
September 19, 2006
DHEA supplements, touted for anti-aging and strength, reviewed by ConsumerLab.com — Testing finds one brand with 215% of the labeled amount
WHITE PLAINS, NEW YORK — SEPTEMBER 19, 2006 — New tests of supplements containing the hormone DHEA found most to contain their claimed amounts of the controversial ingredient, but one provided 215% of its stated amount, the testing company ConsumerLab.com reported today.
Clinical Update
9/30/2022
Fish Oil for Muscle Strength?
Does supplementing with high-dose fish oil improve muscle strength or endurance in older women and men? Find out what a recent study showed in the Muscle, Strength and Falls section of our Fish Oil Supplements Review.
Also see our article about supplements to consider using when exercising (and ones to avoid).
Clinical Update
6/07/2022
Liver Danger With Muscle-Building Supplement
A case of liver toxicity was linked to use of an illegal ingredient found in some muscle-building supplements. Get the details in the Concerns and Cautions section of our Muscle & Workout Supplements Review.
Clinical Update
9/07/2022
Urolithin A for Muscle Strength?
Can supplementing with urolithin A improve strength and endurance in middle-aged women and men? Find out what a recent study showed in our updated CL Answer about urolithin A for age-related muscle decline.
Also see our answer to the question: What's the best protein to keep and gain muscle when you're older?
Clinical Update
10/03/2023
Reducing Age-Related Muscle Loss
Do any supplements help build and maintain muscle and strength as we age? Find out in our article about supplements and age-related muscle loss.
Clinical Update
3/21/2023
Acai for Exercise Recovery?
Did taking an acai supplement reduce muscle fatigue and soreness after exercise? Find out what a recent study showed in the What It Does section of our Acai Berry Supplements & Beverages Review.
Also see: Do any supplements, foods, or oils help reduce muscle pain, muscle cramps, or nighttime leg cramps?
Clinical Update
4/07/2022
Pickle Juice for Muscle Cramps?
Can drinking pickle juice help relieve muscle cramps and are there safety concerns? Find out in our updated article about Supplements for Muscle Cramps.
Clinical Update
4/19/2022
Pickle Juice Works for Muscle Cramps
A recent study found that pickle juice reduced the severity of cramps (e.g., in the calves and feet). Get the details, including the dosage, timing, and type of pickle juice used in our article about Supplements for Muscle Cramps.
Also find out if a product called HOTSHOT might be beneficial for muscle cramps.
Clinical Update
4/26/2022
Krill Oil & Muscle Strength
Supplementing with krill appeared to improve muscle strength in older men and women in a recent study. See the Muscle, Strength and Falls section of our Fish Oil Supplements Review for details.
Clinical Update
2/23/2022
Leucine for Muscle Loss?
Did leucine supplementation improve physical function in people ages 70 and older with age-related muscle loss in a recent study? Find out in the Branched-chain amino acids (BCAAs) section of our Muscle & Workout Supplements Review.
Also see our answer to the question: Which vitamins and minerals should someone over 70 take?
Clinical Update
2/25/2022
HMB for Muscle Loss in Older People?
Can HMB supplementation improve muscle mass and strength in older people? Find out what research suggests, including who is most likely to benefit, in the HMB section of our Muscle & Workout Supplements Review.
Clinical Update
6/19/2021
Glutamine and Exercise
Can glutamine supplementation decrease muscle breakdown from exercise? See what a recent study showed in the What It Does section of our Muscle & Workout Supplements Review. Also see our Top Picks among muscle and workout products.
Clinical Update
12/22/2018
Curcumin for Muscle Soreness
Does curcumin reduce muscle inflammation or soreness from intense exercise? Find out what recent studies have shown in the Muscle Soreness and Recovery After Exercise section of the Turmeric and Curcumin Supplements & Spices Review. (Also see our Top Picks among products.)
Clinical Update
8/27/2019
Whey Protein for Muscle Gain and Fat Loss?
Did drinking whey protein after resistance exercise help prevent muscle loss and reduce body fat in obese women? See what a recent study found in the Building and maintaining muscle section of the Protein Powders, Shakes and Drinks Review. Also see our Top Picks for protein powders and shakes.
Clinical Update
1/12/2020
Collagen vs. Whey for Muscle
Does taking collagen protein increase muscle as well as whey protein? Find out what a recent study showed in the "Building Muscle" sections of both the Protein Powders Review and the Collagen Supplements Review. Also see our Top Picks for protein powders and Top Picks for collagen.
Clinical Update
7/23/2017
Does MSM Help Joints and Muscles?
MSM (methylsulfonylmethane) is commonly in supplements for joint and muscle pain, although the evidence behind it has been limited. Its effectiveness in reducing pain, muscle damage, and oxidative stress was recently evaluated in people running a half-marathon. See the results in the "What It Does" section of the Joint Health Supplements Review (Glucosamine, Chondroitin, MSM, and Boswellia), which includes our Top Picks among products.
Clinical Update
4/28/2017
Vitamin D for Muscle and Balance?
Why did a recent study show that both high and low-dose vitamin D didn't improve muscle strength or balance in women despite the fact that other studies have shown vitamin D to help? The likely answer has to do with the vitamin D status (i.e., blood levels) of the people chosen to participate in these studies. To find out what you should do to improve your balance and reduce your chance of falling, see the "Muscle, Balance and Falls" section of the Vitamin D Supplements Review >>
Clinical Update
6/23/2018
Vitamin D for Strength?
Muscles contain vitamin D receptors, but does giving high-dose vitamin D to women deficient in vitamin D improve their muscle strength? Get the answer in the recent update to the Muscle, balance and falls section of the Vitamin D Supplements Review.
Clinical Update
7/24/2018
Vitamin D and Leg Muscle Strength
Giving a moderate amount of vitamin D to women deficient in vitamin D was shown to improve leg muscle strength. A much higher dose did not. Get the details, including dosage, and learn about vitamin D for strength and balance in the Muscle, Balance, and Falls section of the Vitamin D Supplements Review. (Also see our Top Picks among vitamin D supplements.)
Clinical Update
12/25/2018
Vitamin D for Muscle
Vitamin D increased muscle mass in older men and women with low levels of vitamin D in a recent study. Get the details in the Muscle, balance and falls section of the Vitamin D Supplements Review. (Also see our Top Picks for vitamin D.)
Clinical Update
1/12/2019
Maintaining Muscle With Fish Oil?
Can taking fish oil prevent muscle loss when a limb is immobilized, such as in a brace? Find out what a recent study showed in the "Strength and Muscle" section of the Fish Oil Supplements Review. (Also see our Top Picks for fish oil.)
Clinical Update
4/15/2015
Muscle Supplements and Testicular Cancer
A study of men who have used muscle-building supplements shows a disturbing increase in the risk of testicular cancer. More information is in the Muscle Enhancers (Creatine and Branched-chain Amino Acids) Review >>
Clinical Update
9/02/2015
Vitamin D Increases Strength
A recently published review of clinical studies found that supplementing with vitamin D can improve muscle strength in young, healthy men and women. This has also been found in older individuals and may relate to the fact that vitamin D receptors occur in skeletal muscle. However, this benefit may be limited to vitamin D deficient individuals. Get the details in the "Muscle, balance and falls" section of the Vitamin D Supplements Review >>
Clinical Update
1/24/2016
Leucine Protects Muscle In Middle-Aged
A recent study found that active, middle-aged people restricted to bed rest for several days lost significantly less muscle mass and function if given a leucine supplement along with their meals. Fat gain was also reduced. Get the details, including our tests of leucine-containing supplements, in the Muscle Enhancers Review >>
Recalls & Warnings
March 21, 2024
Eleven Brands of Sexual Enhancement Supplements Recalled Due to Undeclared Prescription Drugs
On March 19, 2024, Pyramid Wholesale issued a recall of 11 brands of sexual enhancement supplements, including honey-based products, after they were found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
May 08, 2017
Muscle Enhancement Supplement Recalled
On May 5, 2017, Genetic Edge Compounds issued a recall of all lots its muscle enhancement supplement GEC Laxoplex because FDA analysis found it to contain anabolic steroids and steroid like substances, which are not permitted in dietary supplements.
Recalls & Warnings
April 05, 2016
Muscle Enhancement Supplement Recalled
On April 5, 2016, Invisiblu International LLC issued a recall of one lot of the muscle enhancement supplement Continuum Labs LGD-Xtreme because it contains LGD-4033 Ligandrol. The risks of this ingredient are not known.
Recalls & Warnings
September 26, 2015
Seller of Muscle Supplements and More Warned for Manufacturing Violations
On August 14, 2015, the FDA issued a warning letter to Chaotic Labz, Inc.
Recalls & Warnings
November 14, 2014
FDA Warns Consumers Not to Buy or Use Muscle Enhancement Supplement
On November 13, 2014, the FDA advised consumers not to buy or use the muscle enhancement supplement Mayhem (Chaotic-Labz) because it was found to contain the undeclared drugs cyproheptadine and dexamethasone. These drugs can cause serious side effects and can interact with other medications.
Recalls & Warnings
June 12, 2024
Integrity Enhancement Supplements for Men Recalled Due to Undeclared Prescription Drugs
On February 1, 2024, Integrity Products issued a recall of one lot of Ram It & To The Moon products, which are promoted for sexual enhancement, after FDA analysis found the capsules to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
November 22, 2017
FDA Warns Distributor of "Testosterone Wellness for Men"
On November 8, 2017, the FDA sent a warning letter to Vita-Pure, Inc.
Recalls & Warnings
April 03, 2024
ForeverMen Sexual Enhancement Supplements Recalled Due to Undeclared Prescription Drugs
On February 12, 2024, ForeverMen issued a recall of all lots of ForeverMen capsules, which are promoted for sexual enhancement, after FDA analysis found the capsules to contain undeclared sildenafil and tadalafil.
Clinical Update
10/10/2020
Magnesium for Muscle Soreness?
Can magnesium supplementation decrease muscle soreness after exercise? See what a new study found in the What It Does section of Magnesium Supplements Review. Also see our Top Picks for magnesium.
Clinical Update
8/21/2021
Omega-7s for Muscle Pain?
Can supplementation with omega-7 fatty acids (from fish oil) reduce muscle pain or inflammation in the body? See what a new study found, and learn if omega-7s have other benefits in the omega-7 section of our Fish Oil Supplements Review. Also see how much omega-7 we found in popular fish oil products.
Clinical Update
11/04/2020
Muscle Cramps from Lack of Vitamin
Painful muscle cramps in an older person resolved after correcting a deficiency in one of the B vitamins, according to a new report. Get the details, and learn which conditions and medications increase the risk of this deficiency, in the B Vitamin Supplements Review. Also see our Top Picks for B vitamin supplements.
Clinical Update
12/06/2020
Do Creatine or BCAAs Boost Strength?
When coupled with resistance exercise, does supplementation with creatine or branched chain amino acids (BCAAs) further boost muscle strength? Find out what separate recent studies showed for creatine and BCAAS in the "What It Does" section of our Muscle & Workout Supplements Review. Also, see our Top Pick for creatine and BCAA supplements.
Clinical Update
5/19/2013
Creatine for Fibromyalgia
A new study shows that creatine might have a role in boosting muscle strength in patients with fibromyalgia. Get the details, plus quality ratings for creatine supplements in our updated Muscle Enhancers (Creatine and Branched Chain Amino Acids) Review >>
Clinical Update
12/09/2012
Extra Protein Improves Strength
Whether you are trying to add muscle or just maintain it, a new report suggests that additional protein leads to further gains from resistance-type training. The analysis of 22 clinical studies shows that both young and old individuals who received additional protein from supplements or high-protein foods experienced significantly greater increases in muscle mass and strength than those not getting additional protein. Get the details, as well as our tests and reviews of protein powders and drinks, in the updated Protein Powders and Drinks Review (Including Sports, Nutrition, and Diet Products). More >>
Clinical Update
3/22/2016
Curcumin for Muscle Soreness?
Some studies show that curcumin (from turmeric) can reduce muscle soreness after exercise. However, a more recent study failed to show this benefit. Get the details, including the dose and brand of curcumin used, in the "What It Does" section of the Turmeric and Curcumin Supplements & Spices Review >>
Clinical Update
10/27/2015
Whey Protein and Muscle
As noted in our Protein Review, many studies show that extra protein can help build more muscle and increase strength when resistance training. But, as shown in a recent study in postmenopausal women, just eating extra protein without exercise provides no benefit. Get the details in the Protein Powders and Drinks Review >>
Clinical Update
4/15/2015
Vitamin D and Statin-Related Muscle Pain
Taking high doses of vitamin D may reduce statin-related muscle pain in people with low blood levels of vitamin D, according to a new study. Get more information and our test reports on vitamin D supplements in the Vitamin D Supplements Review >>
Clinical Update
5/24/2015
Fish Oil Increases Muscle/Strength
Last week we reported that vitamin D could help increase strength -- this week it's fish oil. A recent study found that taking a high dose of fish oil led to increases in muscle and strength in older individuals -- without additional exercise. As reported earlier, a lower of dose of fish oil may improve results of exercise. For the details, including specifics about the fish oil used, see the Fish Oil Supplements Review >>
Clinical Update
3/09/2011
Fish Oil Helps in Chemotherapy
A recent study in lung cancer patients showed that those using fish oil supplementation during initial chemotherapy tended to maintain their muscle and total weight while those who did not lost 5 lbs of weight on average (2.2 lbs of which was muscle). See the Fish Oil (Omega-3) Product Review for more details, including the dosage used. More >>
Clinical Update
11/10/2013
Amino Acids Speed Recovery From Knee Surgery
A study of men and women receiving knee replacement surgery showed that taking essential amino acids (including branched-chain amino acids) twice daily before and after surgery reduced muscle loss and speeded recovery. For details, including dosage, plus our reviews of amino acid supplements, see the updated Muscle Enhancers Review >>
Clinical Update
11/26/2014
CoQ10 Reduces Statin-Related Muscle Pain
Taking a moderate dose of CoQ10 reduced statin-related muscle symptoms in 75% of patients in a recent study. More details, as well as our tests of CoQ10 and ubiquinol products, are found in the CoQ10 & Ubiquinol Supplements Review >>
Clinical Update
1/28/2015
Pea Protein for Muscle
Like whey protein, pea protein is now being used in protein powders. A recent placebo-controlled study compared the two and suggests that pea protein is a viable option for increasing muscle mass. See the details in the Protein Supplements Review >>
Clinical Update
11/28/2017
Curcumin for Muscle Soreness
Another study was published recently suggesting that curcumin can reduce muscle soreness after exercise. However, the efficacy of curcumin for this use is questionable. For details, see the "What It Does" section of the Turmeric and Curcumin Supplements Review >> (Also see our Top Picks for turmeric and curcumin supplements.)
Clinical Update
8/12/2015
Curcumin May Reduce Muscle Pain After Exercise
A small study has reported that curcumin reduced muscle pain after exercise in young, healthy men. Get the details, including dosage, plus more about curcumin for other types pain and our tests of popular products, in the Turmeric and Curcumin Supplements Review >>
Clinical Update
9/25/2018
Extra Protein for Extra Muscle?
Extra protein can boost the increases in muscle strength and size that occur with strength training. But does extra protein help people already consuming adequate protein from their diets? See what a new study found in the What It Does section of the Protein Supplements Review. (Also see our Top Picks among protein powders and shakes.)
Clinical Update
10/30/2018
CoQ10 & Statin Muscle Symptoms
Can taking CoQ10 reduce muscle symptoms that can occur when taking cholesterol-lowering statin drugs? Find out in the recent update to the What It Does section of the CoQ10 & Ubiquinol Supplements Review. (Also see our Top Picks for CoQ10 and Ubiquinol.)
Clinical Update
4/04/2017
Curcumin for Muscle Soreness?
Several studies have evaluated curcumin to reduce muscle soreness, pain, and loss of power after exercise. The most recent study showed little benefit. For details, see the "What It Does" section of the new Turmeric/Curcumin Supplements & Spices Review >>
Clinical Update
2/10/2018
Protein Enhances Muscle Gain in Young and Old
Consuming extra protein, up to a point, was found to help both younger and older people increase muscle strength and size as part of resistance exercise training. For details see the What It Does section of the Protein Powders Review. (Also see our Top Picks among protein powders).
Clinical Update
8/18/2018
Extra Protein Can Help Older People
Increasing protein intake can help older people maintain and build muscle — but it's not that simple. Get the details, and read about the latest studies, in the Building and maintaining muscle mass section of the Protein Supplements Review. (Also see our Top Picks among protein shakes and drinks).
Clinical Update
10/05/2018
Amino Acids After Knee Replacement?
Does taking amino acids after knee replacement surgery help maintain muscle volume, mobility, and strength? See what a recent study found in the What It Does section of the Muscle & Workout Supplements Review. (Also see our Top Picks in this category).
Clinical Update
12/18/2018
Creatine for Older People
Does taking creatine improve muscles in older people? See the results of a recent study in the What It Does section of the Muscle & Workout Supplements Review. (Also see our Top Pick for creatine).
Clinical Update
1/14/2017
Enzymes for Muscle Soreness
A blend of enzymes reduced delayed-onset muscle soreness after exercise in healthy men. For details, see the "What They Do" section of the Digestive Enzyme Supplements Review >>
Clinical Update
11/19/2019
When Protein Won't Help
Supplementing with protein can help build and maintain muscle mass, but there are times when it won't help, as demonstrated in a recent study. For details see the Building and maintaining muscle mass section of the Protein Powders Review. Also see our Top Picks for protein supplements.
Clinical Update
11/29/2019
A Better Form of Creatine?
A relatively new form of creatine may raise creatine levels in the blood and muscle more effectively than the most common form (creatine monohydrate), but it has drawbacks. Learn more in the ConsumerTips section of the Muscle & Workout Supplements Review. Also see our Top Picks for creatine.
Clinical Update
6/21/2022
Muscle Weakness from Mushrooms?
Be aware that, although rare, mushrooms and mushroom supplements may trigger a rare autoimmune reaction resulting in muscle pain or weakness. Get the details in the Concerns and Cautions section of our Reishi Supplements Review.
Clinical Update
9/02/2022
CoQ10 for Statin-Related Muscle Pain?
Can CoQ10 supplementation reduce muscle pain in people taking cholesterol-lowering statin medication? See what a recent study found in the What It Does section of our CoQ10 and Ubiquinol Supplements Review.
Also see: When taking a statin drug like Lipitor or Crestor, are there supplements I should avoid or take?
Clinical Update
11/29/2022
Vitamin D & Statin-Related Muscle Pain
Does taking vitamin D reduce the risk of statin-related muscle pain? Find out what a recent study found in the Statins section of our Vitamin D Supplements Review.
Also, see our article about supplements to take or avoid when taking statins.
Clinical Update
1/24/2023
Exercise While Fasting
To build muscle and burn fat, some people exercise without having eaten since the prior day. Having some protein or amino acids around the time of exercise can help with building muscle – but what’s the best time to do that? Find out what research suggests, in our updated CL Answer about essential amino acids.
Also see: I do moderate exercise for about an hour a few times a week. Which supplements might help me?
Clinical Update
2/28/2023
Vegan Protein for Muscle?
Can vegan protein derived from fungus (sold as Quorn) improve muscle mass and strength as effectively as milk-based protein, and is it safe? Find out in the ConsumerTips section of our Protein Powders and Shakes Review.
Also see our Top Picks among protein powders, including a product suitable for vegans.
Clinical Update
6/27/2023
Vitamin D for Strength?
Does supplementing with vitamin D improve strength and muscle function among older men and women with slightly low or adequate blood levels of vitamin D? Find out what research suggests in the Strength and muscle function section of our Vitamin D Supplements Review.
Also see our Top Picks for vitamin D.
Clinical Update
8/25/2023
Tart Cherry for Sore Muscles?
Did tart cherry extract reduce muscle soreness after exercise in a recent study? Find out, plus, see the evidence for tart cherry juice and supplements for improving sleep and memory, and other uses, in our article about the health benefits of tart cherry.
Clinical Update
1/30/2024
PQQ for Muscle Strength?
Did taking PQQ (pyrroloquinoline quinone) increase muscle strength or exercise endurance among older men and women? See what a new study found in our PQQ Supplements Review. Also learn if PQQ helps with memory and cognition or has heart health benefits, and see our Top Picks among PQQ supplements.
Clinical Update
2/18/2022
Do Vitamin D & Whey Protein Help After a Stroke?
Can taking vitamin D and whey protein after a stroke help improve muscle strength and physical function? Find out what a recent study suggests in the Muscle, balance and falls section of our Vitamin D Supplements Review.
Also see our article about supplements and stroke risk.
Clinical Update
3/31/2022
Spinach for Muscle Strength?
Can supplementing with spinach extract improve muscle strength in older adults? Find out what a recent study showed in the What They Do section of our Greens and Whole Food Supplements Review. Also see our Top Picks among greens and whole foods products.
Clinical Update
4/26/2022
B-12 for Nighttime Leg Cramps
Raising lower blood levels of vitamin B-12 may help with nighttime leg cramps according to some reports. Get the details in the B-12 section of our B Vitamin Supplements Review.
For information about other supplements that may help, see our answer to the question: Do any supplements, foods, or oils help reduce muscle pain, muscle cramps, or nighttime leg cramps?
Clinical Update
1/25/2022
Urolithin-A – A New Supplement for the Elderly?
Urolithin-A can improve the function of mitochondria (the “powerhouses” inside our cells). Mitochondrial dysfunction has been linked with various age-related diseases. Find out if taking urolithin-A improved muscle function in recent study of elderly people in our new article: Does urolithin A reduce age-related muscle decline?
Recalls & Warnings
June 22, 2023
FDA Warns Warrior Labz for Selling Products with Steroid-Like Substances and Prescription Drugs
On June 12, 2023, the FDA issued a warning letter to Warrior Labz SARMS following inspection of the company’s website and social media, which found that the company sold the following products labeled as containing steroid-like substances known as selective androgen receptor modulators ...
Recalls & Warnings
February 07, 2024
Sustain and Schwinnng Male Enhancement Supplements Recalled
On February 2, 2024, Today the World issued a recall of all lots of Sustain herbal dietary supplement capsules and one lot of Schwinnng capsules after FDA analysis found the supplements to contain undeclared prescription drugs tadalafil and nortadalafil.
Recalls & Warnings
July 01, 2022
Male Enhancement Supplements Sold on Amazon Recalled
On January 27, 2022, Loud Muscle Science LLC issued a voluntary recall of various lots of Launch Sequence supplements because they were found to contain the prescription drug Tadalfil.
Clinical Update
3/22/2022
Fish Oil: Cognitive Benefit in Healthy People?
Does fish oil supplementation improve cognition in healthy people? See what a recent study showed in the Memory Enhancement in Healthy Individuals section of our Omega-3 Fatty Acid Supplements Review.
Also see our answer to the question: Do any supplements or foods help with brain function, like memory and cognition?
Clinical Update
11/17/2023
Calanus Oil for Memory?
Does supplementing with calanus oil, a source of omega-3 fatty acids, improve memory in older men and women? Find out what research suggests in the Memory Enhancement in Healthy Individuals section of our Fish Oil Supplements Review.
Also see: Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
Clinical Update
3/05/2024
Fish Oil for Memory?
Did taking fish oil improve cognitive function or memory among older people with depression in a recent study? Find out in the Memory Enhancement section of our Fish Oil Supplements Review, which includes our Top Picks for fish oil.
Also see: Do any supplements, foods or lifestyle modifications help with brain function, like memory and cognition?
Clinical Update
2/04/2020
Problems With Huperzine A
A recent analysis of supplements claiming to contain huperzine A (promoted for memory enhancement) found problems with most products. For details see the Concerns and Cautions section of the Huperzine A Supplements Review. Also learn what clinical studies have shown with huperzine A in the What It Does section and see which huperzine A supplements have been Approved by CL.
Clinical Update
8/28/2020
Concern Over Memory Supplement Quality
Many supplements with phosphatidylserine – promoted for memory enhancement -- do not contain their listed amounts of this ingredient, according to a new report. Get details and learn what clinical studies show with phosphatidylserine in our updated answer to the question: Do either phosphatidylserine or phosphatidylcholine help with memory and cognition?
Clinical Update
10/08/2019
Fish Oil to Improve Cognition?
See the results of the most recent study of fish oil to improve memory and cognition in the Memory Enhancement section of the Fish Oil Supplements Review. Also see our Top Picks for fish oil.
Clinical Update
9/30/2015
Inaccurate Yohimbe Labels
Yohimbe supplements contain an active compound, yohimbine, which is an old drug for erectile dysfunction. A recent study found that most yohimbe supplements don't list the amount of yohimbine they contain, and those that do are typically inaccurate — similar to what ConsumerLab.com has found. For more about the study as well as our own tests of products, see the Sexual Enhancement Supplements Review >>
Clinical Update
9/17/2019
Omega-3s for Cognition?
Several studies have attempted to boost cognition and memory in healthy people using omega-3 supplements from fish or krill oil. Find out if they succeeded, including a recent study among adolescents, in the Memory Enhancement in Healthy Individuals section of the Fish Oil Supplements Review. Also see our Top Picks among products.
Clinical Update
6/24/2022
Placebo Effect With Creatine?
Creatine seems to improve strength and endurance during high-intensity exercise, but the benefit may be due to a psychological effect, according to a recent study. Learn more in the What It Does section of our Muscle & Workout Supplements Review.
Clinical Update
9/30/2022
Glutamine Side Effects
A CL Member experienced dizziness, nausea, and anxiety after taking L-glutamine. Find out if these are known side effects of L-glutamine, and learn about other potential adverse effects of glutamine in the Concerns and Cautions section of our Muscle & Workout Supplements Review.
Also see our article about supplements that may cause dizziness.
Clinical Update
7/22/2022
Does Arginine Improve Blood Flow?
L-arginine is often promoted to improve blood flow to muscle tissue, and so is L-citrulline (which is converted to L-arginine in the body). But a recent study adds more evidence that this is often not the case. See the details in the What It Does section our L-Arginine Product Review.
Clinical Update
2/22/2023
Turmeric for Knee Pain?
Did a turmeric extract without curcumin relieve knee pain following stair climbing exercise? Find out what a recent study showed in the Muscle Soreness and Recovery After Exercise section of our Turmeric and Curcumin Supplements Review. Also see our Top Picks among turmeric and curcumin supplements.
Clinical Update
2/28/2023
Fish Oil for Strength?
Does supplementing with fish oil boost gains from resistance exercise? Find out what a recent study suggests in the Muscle, Strength and Falls section of our Fish Oil Supplements Review. Also see our Top Picks for fish oil.
Clinical Update
1/20/2023
Creatine & Weight Gain?
Does supplementation with creatine cause water retention or weight gain? Find out what a recent study suggests in the updated Concerns and Cautions section of our Muscle & Workout Supplements Review. Also see our Top Picks for creatine and BCAA workout supplements.
Clinical Update
1/13/2023
Urolithin A for Osteoarthritis?
Can taking urolithin A reduce symptoms of osteoarthritis? Find out what the latest research suggests in our answer about urolithin A which also discusses its use in reducing age-related muscle decline.
Clinical Update
11/18/2022
Statins & Vitamin D
Can taking vitamin D reduce statin-related muscle pain? See what a recent study found in the What It Does section of our Vitamin D Supplements Review.
Also, see our article about supplements to take or avoid when taking statins.
Clinical Update
10/21/2022
Testosterone Boosters
Many supplements promise to increase testosterone, boost libido, and increase muscle size, but, as ConsumerLab explains, the actual effectiveness of these supplements is limited, and some supplements may actually reduce testosterone levels. Get the details in our new article 7 Supplements That May Boost Testosterone (And 7 That May Not).
Clinical Update
10/18/2022
High-Intensity Training + Protein?
High-intensity interval training (HIIT) is a popular way to work out, but does taking a protein supplement after HIIT increase muscular benefits? Find out what a recent study showed in the Building and maintaining muscle mass section of our Protein Powders and Shakes Review.
Clinical Update
3/05/2024
Problems with Creatine
Recent tests of lesser-known brands of creatine gummies revealed that half contained little creatine and/or had impurities. Get the details by brand in our Muscle Enhancers Supplements Review. Also see our Top Picks among creatine supplements.
Clinical Update
12/19/2023
Vitamin D & Statin Side Effects
Does taking vitamin D prevent or reverse statin-related muscle symptoms? Find out what a recent study showed in the Statins section of our Vitamin D Supplements Review, which includes our Top Picks for vitamin D.
Clinical Update
11/10/2023
Creatine for Brain Health?
Creatine, a common workout supplement, is also being used to boost memory and cognition, but does it work? Find out what research shows in the Creatine for Memory section of our Muscle & Workout Supplements Review, which includes our Top Picks for creatine.
Clinical Update
6/27/2023
Does Omega XL Work?
We’ve updated our article about this supplement widely promoted for joint health and muscle recovery. See our updated information about Omega XL.
Clinical Update
8/18/2023
Fenugreek for Strength?
Does supplementing with fenugreek enhance the benefits of resistance exercise? Find out what a recent study showed in our article about fenugreek.
Also see our Muscle & Workout Supplements Review.
Clinical Update
9/26/2023
Creatine for Post-COVID Fatigue?
Does supplementing with creatine reduce fatigue or other symptoms in people with post-COVID fatigue syndrome? Find out what a recent study showed in the Creatine - What It Does section of our Muscle & Workout Supplements Review. Also see our Top Picks for creatine.
Also see: How Should You Take Care of Yourself After You’ve Had COVID-19?
Clinical Update
9/26/2023
Vitamin D for Strength?
Does supplementing with vitamin D during winter improve strength among people with low levels of vitamin D? Find out what a recent study showed in the Strength and muscle function section of our Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
3/28/2023
Protein For Faster Improvement?
Did taking a protein supplement lead to faster improvement in walking speed among older men and women than exercise alone? Find out what a recent study showed in the Building and maintaining muscle mass section of our Protein Supplements Review.
Also see our Top Pick among protein supplements (including whey, casein, pea, and soy).
Clinical Update
3/28/2023
BCAAs for Post-surgery Recovery?
Does taking branched-chain amino acids (BCAAs) after lower back surgery help with recovery? Find out what a recent study showed in the BCAAs section of our Muscle & Workout Supplements Review.
Also see our Top Picks among BCAA supplements.
Clinical Update
3/31/2023
Hyland's for Leg Cramps?
Is there good evidence that Leg Cramps by Hyland’s Naturals relieves cramps in the legs and feet? Find out in the Supplements and creams for muscle cramps section of our article about leg cramps.
Clinical Update
4/07/2023
Collagen Versus Whey Protein?
Find out if taking collagen (which is a protein) improves wrinkles if you already use a protein powder, such as whey. Also, learn if collagen is as good as whey for muscle maintenance.
Clinical Update
6/06/2023
CoQ10 for Strength?
Does supplementing with the ubiquinol form of CoQ10 improve muscle strength in well-trained men? Find out what a recent study showed in the Strength and athletic performance section of our CoQ10 Supplements Review.
Also see our Top Picks for CoQ10 and ubiquinol.
Clinical Update
7/22/2024
CoQ10 for Statin-Related Weakness?
Cholesterol-lowering statin drugs can cause weakness in some people. Lowering the statin dose can sometimes help, and doing so along with taking CoQ10 may be even more helpful according to a recent study. Get the details in the Muscle pain section of our CoQ10 and Ubiquinol Supplements Review, which includes our Top Picks for CoQ10.
Clinical Update
2/11/2022
Omega-3s for Fall Prevention?
Can omega-3 supplementation help reduce falls in older men and women? See what a study found in the Muscle, Strength and Falls section of our Fish Oil/Omega-3 Fatty Acid Supplements Review. Also, see our Top Picks among fish oil supplements.
Clinical Update
3/31/2022
Quinine for Leg Cramps?
Is quinine (a compound in tonic water) helpful and safe for nighttime leg cramps? Find out in our updated answer about supplements for leg cramps and muscle pain.
Clinical Update
3/01/2022
Does Boosting Protein Intake Help?
Can getting extra protein boost physical function or improve body composition in older people? See what a recent study showed in the Building and maintaining muscle mass section of our Protein Supplements Review. Also see our Top Picks among protein supplements.
Clinical Update
3/22/2022
CoQ10 for Statin Side Effects?
Does CoQ10 reduce muscle side effects of statin drugs (used to lower cholesterol)? See what a recent study found in the What It Does section of our CoQ10 Supplements Review.
Clinical Update
5/10/2022
Choosing a Tart Cherry Product
Which tart cherry products are best for reducing muscle pain, improving sleep, or lowering blood pressure or cholesterol? Although the evidence is not strong, we identified products with the most evidence behind them in our Tart Cherry article.
Clinical Update
4/22/2022
Does mustard help with cramps?
Several CL members have reported that taking mustard has relieved muscle cramps. Find out why this may help in our answer about foods and supplements for cramps.
Clinical Update
3/11/2017
Can High-Dose Vitamin D Backfire?
A recent study found that taking very high doses of vitamin D increases the production of an enzyme which inactivates vitamin D in the body -- which may help to explain why taking very high-dose vitamin D has been linked with adverse effects such as muscle weakness and increased risk of fractures and falls in previous studies. For details, see the "How Much Do You Need?" section of the Vitamin D Supplements Review (which includes our tests of products) >>
Clinical Update
2/17/2015
Creatine Tested for Parkinson Disease
Taking creatine daily for at least five years did not slow the progression of Parkinson disease according to a recently reported study involving nearly 1,000 patients. For more details about the study, other studies with creatine (for strength, fibromyalgia, etc.), and our tests of creatine supplements, see the Product Review of Muscle Enhancers (Creatine and Branched-chain Amino Acids) >>
Clinical Update
3/17/2015
Zinc Lozenges for Colds
A recent review of placebo-controlled studies of zinc lozenges given at the start of a cold shows that they not only shorten the duration of symptoms in the throat, but in the nose and muscles as well. It is critical, however, that the right type of zinc, dose, and dosing schedule be used. Get the details, as well as our reviews of zinc lozenges and supplements, in the Zinc Supplements and Lozenges Review >>
Clinical Update
1/18/2014
Vitamin D Helps Fibromyalgia
Fibromyalgia is an inflammation of the muscle and soft tissue which causes pain and fatigue. A recent study found that giving vitamin D reduced the pain associated with fibromyalgia. For details about the dose and target vitamin D blood level in the study, as well as our tests of vitamin D supplements, see the updated Vitamin D Supplements Review >>
Clinical Update
7/05/2014
Curcumin Reduces Soreness After Exercise
Taking curcumin before and after intense exercise significantly reduced muscle soreness in young, healthy men, according to a new study. Get the details, including the brand and dose of curcumin used, plus our tests of curcumin supplements, in the updated Turmeric and Curcumin Supplements and Spices Review >>
Clinical Update
10/08/2014
Energy Drinks: Athletic Performance & Side Effects
A recent study found that a caffeinated energy drink improved athletes' self-perceived muscle power, but also increased insomnia and nervousness. Get the details in the update to the B Vitamin Supplements and Energy Drinks Review >>
Clinical Update
3/11/2012
Strength Improves with Vitamin D
A review of 13 studies found that vitamin D supplements may improve balance and muscle strength, but not gait, among older individuals. See the update to the Vitamin D Supplements Review for details, including dosage. More >>
Clinical Update
2/26/2019
Does CBD Cream Help?
Does CBD cream decrease muscle soreness following exercise? Does CBD gel help with knee pain from osteoarthritis? Learn what recent studies have found in the What It Does section of the CBD and Hemp Extract Review. Also see our Top Picks for CBD oils and topical products.
Clinical Update
5/15/2018
Casein at Night?
It has been suggested that casein protein be taken at bedtime because it is absorbed relatively slowly, providing a more continuous supply of amino acids throughout the night to maintain muscle. A study recently put this theory to the test. See the results in the Casein section of the Protein Powders Review. Also see our Top Picks among products.
Clinical Update
6/15/2018
Better Bone Broth
How a bone broth is prepared can significantly affect its content of amino acids - building blocks for muscle and collagen, according to a recent study. Learn how to find or make better bone broth in the ConsumerTips section of the Bone Broth Review. (Also see our Top Picks among bone broths.)
Clinical Update
1/15/2019
Pea vs. Whey Protein
Which is better for increasing strength and muscle thickness, pea protein or whey protein? See what a recent study showed in the Protein Powders Review. Also see our Top Pick for each type of protein.
Clinical Update
2/02/2019
Whey Protein When Bedridden?
Does substituting whey protein for other sources of protein help bedridden people retain muscle mass and strength? Find out what a new study showed in the What It Does section of the Protein Powders & Drinks Review. Also see our Top Picks for protein powders.
Clinical Update
10/22/2019
Does Magnesium Reduce Exercise Soreness?
Can taking magnesium reduce muscle soreness after exercise? See what a new study found in the What It Does section of the Magnesium Supplements Review. Also see our Top Picks for magnesium supplements.
Clinical Update
12/09/2018
When to Take Protein
Supplementing with protein can increase muscle mass and strength more than resistance exercise alone, but the timing of protein consumption can make a difference if you are also trying to improve metabolism and reduce body fat. Get the details and find out what a new study showed in the What It Does section of the Protein Powders, Shakes & Drinks Review. (Also see our Top Picks among products).
Clinical Update
11/09/2019
CoQ10 for Statin-Intolerance
Many people experience muscle pain from taking cholesterol-lowering statin drugs. CoQ10 can help reduce this pain, according to a new study. Get the details in the What It Does section of the CoQ10 Supplements Review. Also see our Top Picks among CoQ10 supplements.
Clinical Update
7/29/2017
Fish Oil for Endurance
High-dose fish oil may help to increase exercise endurance, according to a new study, adding to evidence of other beneficial effects of fish oil on muscle mass and strength. For details, see the "What It Does" section of the Fish Oil Supplements Review, which includes our Top Picks among products.
Clinical Update
2/10/2016
Extra Protein Helps During Exercise & Dieting
A study among obese young men found that giving extra protein during a period of dieting and intense exercise caused them to lose more fat and gain more muscle than men to whom additional protein was not provided. Several other recent studies in both young and old individuals have shown significant benefits from additional protein – but only when combined with exercise. Get the details in the Protein Powders and Drinks Review >>
Clinical Update
3/17/2018
L-Arginine for Workouts?
A recent study assessed whether taking L-Arginine before a high-intensity workout decreased muscle soreness after the workout. Get the results and more details in the Exercise Endurance and Recovery section of the L-Arginine Supplements Review. (Also see our Top Picks among products).
Clinical Update
4/29/2018
When to Take Protein
When is the best time of day to take a protein supplement if you want to gain muscle but lose fat? An analysis of over 30 clinical studies involving protein supplementation and resistance exercise recently addressed this question. Get the answer and details in the ConsumerTips section of the Protein Powders, Shakes and Drinks Review. (Also see our Top Picks among protein products).
Clinical Update
4/12/2016
Too Much Vitamin D Weakens Legs
In recent months we have reported that giving too much vitamin D to people who don't need it increases the risk of falls. A major study now shows that it also weakens leg muscles — apparently to the surprise of the researchers. Keep in mind that giving vitamin D to people who are deficient in it can decrease the risk of falls. Get the details in the Vitamin D Supplements Review >>
Clinical Update
1/17/2017
Red Yeast Rice Concerns
A review of adverse events associated with the use of red yeast rice products in Italy led researchers to suggest monitoring for signs of muscle and liver injury when using red yeast rice. For more details, see the "Cautions and Concerns" section of the Red Yeast Rice Supplements Review (which includes our tests of products) >>
Clinical Update
5/09/2017
Curcumin and Muscle Soreness
A recent study found a particular curcumin formula helped reduce soreness after exercise – although this has not been the case in other studies with different formulas. Find out what might help you in the "What It Does" section of the Turmeric/Curcumin Supplements Review >>
Clinical Update
5/20/2015
Vitamin D Strengthens Muscle
In a recent study, a modest daily dose of vitamin D led to impressive gains in leg strength in women who were deficient in the vitamin. Details about this study (including dosage) and other studies of vitamin D, along with our reviews of products, are found in the Vitamin D Supplements Review >>
Clinical Update
9/04/2018
Glutamine for IBS?
Glutamine, an amino acid, plays a role in maintaining the digestive tract. Can taking it as a supplement help people with irritable bowel syndrome (IBS)? Find out what a recent study showed in the Glutamine section of the Muscle Enhancers Review.
Clinical Update
3/26/2019
Vitamin C Blunts Exercise Benefits
If you are trying to build muscle with resistance exercise, go easy on vitamins C and E, according to a recent study. Get the details in the Concerns and Cautions section of the Vitamin C Supplements Review.
Clinical Update
6/15/2019
Tart Cherry for Soreness After Exercise?
Can tart cherry juice improve muscle recovery and reduce soreness after exercise? See what a new study found in our update to the question: What are the benefits of tart cherry juice?
Clinical Update
7/26/2019
MCT Oil for Strength?
Does adding MCTs (medium chain triglycerides) to the diets of elderly people enhance the benefit they get from exercise? See what a recently published study found in the What It Does: Muscle Strength and Function section of the Coconut Oil and MCT Oil Supplements Review. Also see our Top Picks for coconut and MCT oils.
Clinical Update
11/12/2019
Help for Jaw Pain?
Can CBD ointment relax and reduce pain in the jaw muscles of people who grind their teeth? See what a recent clinical study found in the What It Does section of the CBD and Hemp Extract Review. Also see our Top Picks among products.
Clinical Update
3/21/2020
When to Eat Protein
Some evidence indicates that it is best to distribute protein across meals throughout the day to maximize protein production in muscle. A recent study suggests that there is one meal when protein intake could be higher for nearly everyone, and there is another meal when older people particularly fall short. For details see the When to Take section of the Protein Powder and Drinks Review. Also see our Top Picks for protein powders and drinks.
Clinical Update
5/05/2020
Does Too Much Vitamin D Hurt Muscle?
A study among women given moderately high doses of vitamin D found that they lost strength. Further analysis of the data suggests why. Get the details in the What It Does section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
7/02/2017
Extra Protein Enhances Training
Even among untrained, middle-aged men, adding protein supplementation to resistance training leads to greater improvements in lean body mass (i.e., more muscle) than the training alone, according to a new study. Find out how much protein helped in the "What It Does" section of the Protein Supplements Review. The Review includes our test-based, quality ratings and comparisons of popular protein powders and drinks.
Clinical Update
3/10/2020
Does CoQ10 Help With Statins?
Many studies have looked at whether or not CoQ10 helps people with statin-associated muscle pain. Read the latest in the What It Does section of the CoQ10 and Ubiquinol Supplements Review. Also see our Top Picks for CoQ10 and ubiquinol supplements.
Clinical Update
4/21/2020
Does Protein Powder Affect Blood Flow?
Does supplementing with whey protein isolate affect blood flow through the aorta and to the brain? Find out what a recent study found in the What It Does section of the Protein Powders & Drinks Review. Also learn what effects protein powder has on building and maintaining muscle and see our Top Picks for protein powders.
Clinical Update
3/14/2021
Fibromyalgia Supplement?
Pycnogenol (French pine bark extract) was recently reported to reduce muscle pain and fatigue in people with fibromyalgia, but how strong is the evidence?
Clinical Update
4/09/2021
Vitamin D & Muscle
Can taking high-dose vitamin D improve strength gains from exercise? See what a new study found in the What It Does section of our Vitamin D Supplements Review. Also see our Top Picks among vitamin D supplements.
Clinical Update
12/09/2020
Too Much Vitamin D
A 2-year study suggests risks with vitamin D at daily doses of 1,000 IU and higher. Get the details in the Muscle, balance and falls section of the Vitamin D Supplements Review. Also see our Top Picks for vitamin D.
Clinical Update
5/25/2021
Does Creatine Cause Hair Loss?
Find out if creatine supplements cause hair loss. See the Concerns and Cautions section of our Muscle & Workout Supplements Review. Also, see our Top Picks for creatine supplements.
Clinical Update
6/15/2021
Vitamin D May Increase the Risk of Fractures from Falls
Taking doses of vitamin D above the RDA when not necessary may increase the risk of first-time falls with fractures, according to a recent analysis. Get the details in the Muscle, Balance and Falls section of the Vitamin D Supplements Review. Also see our Top Picks for low, moderate and high-dose vitamin D supplements.
Clinical Update
9/14/2021
Citrulline for Strength?
Find out if citrulline supplementation improved muscle strength or endurance in older people in recent study. (This information is in the Citrulline section of our L-Arginine Supplements Review, as citrulline is converted to arginine in the body and it is often sold in combination with arginine).
Clinical Update
10/19/2021
Alpha Lipoic Acid for Pain?
Can taking alpha-lipoic acid decrease nerve, muscle or joint pain? See what a new study found in the What It Does section of our Alpha Lipoic Acid Review. Also see our Top Picks for alpha-lipoic acid.
Clinical Update
1/11/2022
Soy Versus Whey Protein
Can soy protein help increase muscle mass and strength as well as the same amount of whey protein? See what a recent study showed in the Soy section of our Protein Powders and Drinks Review, which includes comparisons of whey, casein, soy, rice, pea and other forms of protein. Also see our Top Picks among protein supplements.
Clinical Update
7/13/2021
Magnesium for Leg Cramps?
Can magnesium supplementation reduce leg cramps? See what a recent analysis of the data showed for leg cramps in pregnant women. Also see the evidence for use by older men and women in our Magnesium Supplements Review.
Also see our answer to the question: Do magnesium creams, sprays and oils help with muscle pain and cramps? How about supplements?
Clinical Update
8/10/2021
Vitamin D & Risk of Falls
A recent study showed that high-dose vitamin D may increase the risk of falls for certain people. Get the details in the Muscle, Balance, and Falls section of our Vitamin D Supplements Review.
Recalls & Warnings
February 15, 2013
Energy Drink Recalled Due To Bacterial Contamination
On December 21, 2012, NBTY issued a voluntary recall of the energy supplement MET-Rx Extreme Thermo Rage Watermelon 8 fl. oz. (Manufactured by MET-Rx Nutrition, Inc.) due to bacterial contamination.
Recalls & Warnings
January 03, 2024
FDA Warns Amazon for Selling Men’s Supplements Containing Prescription Drugs
On December 20, 2023, the FDA issued a Warning Letter to Amazon.com, Inc.
Recalls & Warnings
November 11, 2014
Seller of Sexual, Muscle Enhancement Supplements Warned for Manufacturing Violations and Drug Claims
On August 26, 2014, the FDA issued a warning letter to GE Pharma LLC following a facility inspection which found the company's products, including Fire Burn, Fire Storm, Creatine, Amino Fire, Nitric Fire, Jet Fire, Oxy Fire, HGH, Cissus, Hydro shield, Performa-Test, Raspberry Ketones, Fire Drol, ...
Recalls & Warnings
August 25, 2023
FDA Warns Consumers Not to Use Big Guys Male Energy Supplement
On August 22, 2023, the FDA warned consumers not to buy or use BIG GUYS Male Energy Supplement after FDA laboratory analysis found it to contain undeclared sildenafil.
Recalls & Warnings
September 06, 2023
WEFUN Capsules Recalled Due to Undeclared Sildenafil
On August 25, 2023, WEFUN Inc. issued a recall of 300 boxes of WEFUN Capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
November 30, 2023
Original The Rock Capsules Recalled Due to Undeclared Sildenafil
On October 18, 2023, Noah’s Wholesale, LLC issued a nationwide recall of one lot of the company’s Original The Rock capsules after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
News Release
August 15, 2006
Over 70% of herbal sleep supplements fail tests for quality by ConsumerLab.com — Results for 16 valerian products released today
WHITE PLAINS, NEW YORK — AUGUST 15, 2006 — Most of the valerian dietary supplements recently tested by ConsumerLab.com were low in potency and/or contaminated, the testing company reported today. Valerian, an herbal sleep aid, accounts for approximately $50 million in sales in the U.S.
Recalls & Warnings
July 26, 2023
Gadget Island, Inc. Male Enhancement Supplements Found to Contain Prescription Drugs
On July 21, 2023, the FDA issued a warning letter to Gadget Island, Inc.
Recalls & Warnings
November 17, 2022
FDA Warns Consumers Not to Use Male Enhancement Supplement
On November 10, 2022, the FDA warned consumers not to buy or use Sangter Natural Male Energy Supplement after FDA laboratory analysis confirmed the presence of undeclared sildenafil.
Recalls & Warnings
January 11, 2023
Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 9, 2022, the FDA issued a warning letter to Distributor RFR, LLC after laboratory analysis of the company’s SANGTER Natural Male Energy Supplement found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
January 19, 2023
Male Sexual Enhancement Supplement Adam’s Secret Found to Contain Prescription Medication
On January 10, 2023, the FDA issued a warning letter to HIS Enterprise Inc dba Adam’s Secret USA, LLC after laboratory analysis found Adam’s Secret Extra Strength 3000 Platinum, Adam’s Secret Extra Strength Blue, Adam’s Secret Extra Strength Purple, Adam's Secret ...
Recalls & Warnings
August 03, 2022
Sexual Enhancement Supplement Recalled Due to Sildenafil
On August 1, 2022, DISTRIBUTOR RFR, LLC recalled one lot of SANGTER Energy Supplement 3000 mg to the consumer level after FDA analysis found the product to contain undeclared sildenafil, a prescription medication.
Recalls & Warnings
September 29, 2022
Sexual Enhancement Supplement Sold on Amazon and Walmart Recalled Due to Undeclared Drugs
On September 27, 2022, Proper Trade LLC/My Stellar Lifestyle recalled two lots of Wonder Pill after Amazon laboratory analysis found the product to contain undeclared tadalafil, a prescription medication.
Recalls & Warnings
May 04, 2023
PrimeZEN Enhancement Supplement for Men Found to Contain Prescription Drug
On April 27, 2023, the FDA issued a warning letter to Tager Online, Inc. DBA Volt Candy; Volt Candy Wholesale Club after FDA laboratory analysis determined PrimeZEN Black 6000, a product promoted to improve sexual function for men, contains tadalafil and sildenafil.
News Release
August 01, 2006
ConsumerLab.com evaluates cholesterol-lowering supplements — Test results available for over 90 products; Review of nine popular ingredients
WHITE PLAINS, NY — AUGUST 1, 2006 — ConsumerLab.com has released a major new review of dietary supplements used to improve cholesterol and triglyceride levels.
Recalls & Warnings
August 01, 2013
Fat Loss and Muscle Enhancement Supplements Found To Contain DMAA
On June 17, 2013, the FDA issued a warning letter to Formulife, Inc., dba Purus Labs, Inc., following a facility inspection which found the dietary supplements Fat Smack XR Thermolipolytic, Muscle Marinade Fresh Fruit and Muscle Marinade Cherry Limeade to contain DMAA.
News Release
January 25, 2006
Sexual enhancement supplements analyzed by Consumerlab.com
WESTCHESTER COUNTY, NEW YORK — JANUARY 25, 2006 — ConsumerLab.com announced today that it found only six out of eleven supplements used for sexual enhancement to contain key ingredients listed on their labels and meet other quality criteria.
Recalls & Warnings
July 15, 2024
Hard Steel Supplements for Men Recalled
On July 12, 2024, Supercore Products Group issued a recall of Hard Steel Capsules and Gold Hard Steel Liquid which are used for male erectile dysfunction. The FDA analysis found the capsules to contain sildenafil and acetaminophen.
Recalls & Warnings
January 07, 2017
Seller of Protein and Workout Supplements Warned for Manufacturing Violations, Label Errors
On December 22, 2016, the FDA issued a warning letter to Rock Solid Nutrition, LLC, following a facility inspection which found the company's products, including Whey Isolate (Cinnabun flavor), Pre-Pump (Massive Mango flavor) and Strength Test to be adulterated because ...
Recalls & Warnings
April 05, 2022
Sexual Enhancement Capsules for Men Recalled
On April 1, 2022, F&S Medical Supply, dba Pink Toyz, recalled one lot of Pink Pussycat 3000 mg capsules to the consumer level because FDA analysis found them to contain sildenafil.
Recalls & Warnings
July 15, 2022
Honey-Based Enhancement Supplement Recalled
On July 13, 2022, Shopaax.com issued a recall of all lots of its honey-based sexual enhancement product, Kingdom Honey Royal Honey VIP, after FDA analysis found the presence of undeclared sildenafil.
Recalls & Warnings
July 26, 2022
Sexual Enhancement Supplement Sold on Amazon Recalled
On July 21, 2022, Ultra Supplements LLC issued a recall of one lot of Sustango capsules after Amazon laboratory analysis found the presence of the prescription drug Tadalafil. The company has received no reports of adverse effects related to this recall to date.
Recalls & Warnings
February 13, 2023
FDA Finds Prescription Drug in “100% Natural” Weight Loss Supplement
On February 8, 2023, the FDA warned consumers not to buy or use Alfia 100% Natural Weight Loss Capsules after FDA laboratory analysis confirmed the presence of sibutramine.
News Release
January 03, 2006
Tests of memory enhancing supplements by ConsumerLab.com reveals lead in some ginkgo
WESTCHESTER COUNTY, NEW YORK — TUESDAY, JANUARY 3, 2006 — In its new test report on Memory Enhancement Supplements, ConsumerLab.com has revealed finding significant amounts of lead in certain products on the market.
Recalls & Warnings
July 13, 2022
FDA Warns Sellers of Tainted Honey-Based Sexual Enhancement Products
On July 12, 2022, the FDA issued warning letters to four companies selling honey-based products promoted for sexual enhancement after tests conducted by the FDA found the products to contain the prescription drugs Tadalafil and Sildenafil.
News Release
September 24, 2003
Consumerlab.com finds new forms of creatine prone to problems — Test results of muscular enhancement supplements published online today
WHITE PLAINS, NY — September 24, 2003 — Testing by ConsumerLab.com of creatine supplements found problems with the majority of products sold in liquid, effervescent and chewable forms. Problems were not found among standard creatine "powder" products.
Recalls & Warnings
October 13, 2018
Pharmaceutical Drugs Found In Dietary Supplements Pose Danger to Consumers
Almost 800 dietary supplements sold between 2007 and 2016 contained unapproved pharmaceutical ingredients, according to a study published today in the Journal of the American Medical Association (JAMA).
Recalls & Warnings
February 10, 2022
Three Sexual Enhancement Supplements Sold on Amazon Recalled
On February 8, 2022, specific lots of three male sexual enhancement supplements, Red Mammoth capsules, MAC DADDY RED capsules, MAC DADDY PURPLE capsules, and The Red Pill capsules, were recalled because Amazon laboratory analyses found them to contain undeclared ...
Recalls & Warnings
October 26, 2023
Joint Pain Supplements Recalled Due to Undeclared Drug Ingredients
On October 22, 2023, Botanical-Be recalled of all lots of the company’s Kuka Flex Forte, Artri King, and Reumo Flex capsule products after FDA laboratory analysis found them to contain undeclared diclofenac.
Recalls & Warnings
October 02, 2023
Dangerous Ingredients Found in Ten Arthritis and Pain Supplements
On September 29, 2023, the FDA warned consumers that the following arthritis and pain management products contain undeclared drugs such as diclofenac, methocarbamol (a muscle relaxant), and dexamethasone (a corticosteroid), and/or other potentially harmful ingredients.
Recalls & Warnings
April 17, 2021
NS NY Distributor Inc Male Enhancement Products Recalled Due to Undeclared Drugs
On April 8, 2021, NS NY Distributor Inc issued a recall of all lots of Premium Orgazen 7000 and Ginseng Power 5000 male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
Recalls & Warnings
April 10, 2021
Three More Male Enhancement Products Recalled Due to Undeclared Drugs
Between March 30 and April 5, 2021, three companies issued recalls of their male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
Recalls & Warnings
April 02, 2021
Three Male Enhancement Products Recalled Due to Undeclared Drugs
Between March 24 and 26, 2021, three companies issued recalls of their male enhancement capsules because FDA analysis found them to contain sildenafil and tadalafil.
Recalls & Warnings
February 17, 2021
Adam’s Secret Male Enhancement Products Recalled Due to Undeclared Drugs
On February 15, 2021, adamssecret.co issued a recall of all lots of Adam's Secret Extra Strength 1500 and Adam's Secret Extra Strength 3000 male enhancement capsules because FDA analysis found them to contain sildenafil and/or tadalafil.
Recalls & Warnings
September 03, 2020
Red-E Male Enhancement Capsule Recalled
On September 1, 2020, The Protein Shoppe, LLC issued a recall of Red-E male enhancement capsules because FDA analysis found it to contain sildenafil.
News Release
April 21, 2003
Low quality ingredient appears widespread among Ginkgo supplements according to ConsumerLab.com; points to challenge for FDA's proposed regulations — Review of memory enhancement supplements released today
WHITE PLAINS, NY — April 21, 2003 — In contrast to its findings three years ago, ConsumerLab.com announced today that only 22% of the Ginkgo biloba supplements it recently tested met its quality standards. In late 1999, 75% of the products it tested met these standards.
Recalls & Warnings
December 19, 2020
FDA Finds Unapproved Drugs in Many Weight Loss and Sexual Enhancement Products Sold Online
On December 18, 2020, the FDA warned consumers that certain products promoted for weight loss, body building, sexual enhancement, pain relief, sleep, and other uses because may contain harmful ingredients.
Recalls & Warnings
September 26, 2020
Cognitive Enhancement Supplements Can Contain Unapproved Drugs
Recent testing detected five unapproved drugs in 10 over-the-counter cognitive enhancement and workout supplements sold in the US.
Recalls & Warnings
May 11, 2022
FDA Warns 10 Companies for Selling Workout Supplements With Dangerous Ingredients
On May 4, 2022, the FDA issued warning letters to 10 companies for selling products promoted for muscle building, fat burning and other uses that contain potentially dangerous ingredients not permitted in dietary supplements, including hordenine, higenamine, 5-alpha-hydroxy-laxogenin, and CBD.
Recalls & Warnings
September 28, 2022
FDA Warns Muscle Sports for Joint Health, Immune & Workout Supplement, Vitamin C, Elderberry, and Other Claims
On September 23, 2022, the FDA issued a warning letter to Muscle Sports Products, LLC following inspection of the company’s websites which found statements about company products, including Join Revolution Capsules, IMMUNITY + Powder, Rhino Rampage PUMPED Capsules, Attack Pre-Workout ...
Recalls & Warnings
June 24, 2024
Canned Coffee Recalled Due to Botulism Risk
On June 17, 2024, Snapchill LLC recalled its canned coffee products because they have the potential to grow the toxic bacterium Clostridium botulinum.
Recalls & Warnings
January 15, 2024
5 Star Nutrition Will Pay $4.5 Million for Selling Misbranded Workout Supplements
On January 12, 2024, Defyned Brands, also known as 5 Star Nutrition LLC, pleaded guilty in federal court to three-counts of distributing misbranded dietary supplements following an investigation by the Food and Drug Administration’s Office of Criminal Investigations (FDA-OCI).
News Release
September 24, 2002
Problems found with most sexual enhancement supplements evaluated by ConsumerLab.com — Only 9 of 22 products pass independent review
WHITE PLAINS, NY — September 24, 2002 — ConsumerLab.com announced today that nearly 60% of products failed to pass its independent Product Review of Sexual Enhancement Supplements. Sexual dysfunction is estimated to affect 43% of women and 31% of men in the U.S.
Recalls & Warnings
April 27, 2022
Prescription Drugs Found in Arthritis, Muscle Pain, and Osteoporosis Supplements
On April 20th, 2022, the FDA warned consumers not to use the following ”Artri“ or ”Ortiga” products promoted to treat arthritis, muscle pain, osteoporosis and bone cancer because they have been found to contain undeclared drugs that can have serious adverse ...
Recalls & Warnings
July 14, 2022
FDA Warns Company Selling Supplements with Dangerous Steroid-like Substances
On July 6, 2022, the FDA issued a warning letter to Elite Supplement Center LLC and Elite Supplement Training Facility LLC for selling the following products labeled as containing steroid-like substances known as selective androgen receptor modulators (SARMs), which are not permitted in dietary ...
News Release
May 05, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 5, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
Recalls & Warnings
September 03, 2019
Federal Court Shuts Down Two Supplement Companies Selling Weight, Joint Health and Other Supplements
On September 3, 2019, a U.S. District Court entered a consent decree of permanent injunction against Basic Reset and Biogenyx, two Tennessee-based companies that sell dietary supplements and other product promoted for health benefits.
News Release
April 16, 2002
ConsumerLab.com finds "Breast Enhancement" pills lack evidence of efficacy
WHITE PLAINS, NY — April 16, 2002 — ConsumerLab.com announced today that it found no hard evidence to suggest that current dietary supplements marketed for breast enhancement are effective.
News Release
March 06, 2002
Study finds supplement users avoid prescription drugs due to side effects, not cost — Brands top rated by users also identified in new survey by ConsumerLab.com
WHITE PLAINS, NY — March 6, 2002 — ConsumerLab.com, traditionally known for its independent laboratory evaluations of dietary supplements and nutritional products, announced surprising findings today from a new survey of supplement users.
Recalls & Warnings
October 26, 2019
Green Lumber Enhancement Supplements Recalled
On October 22, 2019, GL Holdings issued a recall of Green Lumber, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain tadalafil.
Recalls & Warnings
September 21, 2019
Liquid Vitamin C for Men Recalled
On September 16, 2019, Fitoterapia USA Inc. issued a recall of Macho Artificial Passion Fruit Flavored Vitamin C Liquid Supplement, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain tadalafil.
Recalls & Warnings
November 16, 2019
Silver Bullet Supplement for Men Recalled
On November 13, 2019, Nature's Rx issued a recall of one lot of Silver Bullet male enhancement capsules because FDA analysis found it to contain sildenafil.
Recalls & Warnings
July 20, 2021
Alpha Male Plus Recalled
On July 16, 2021, Alpha Male Plus issued a recall of Alpha Male Plus Male Enhancer fruit chews because FDA analysis found them to be contaminated with tadalafil.
Recalls & Warnings
April 24, 2023
Terry Naturally and EuroMedica B Vitamins Recalled Due to Allergen Risk
On April 21, 2023, EuroPharma, Inc. issued a voluntary recall of 4 lots of its Terry Naturally BioActive Vitamin B 60 count and 3 lots of its EuroMedica Active B Complex 60 count products due to undeclared milk. No illnesses have been reported to date.
Recalls & Warnings
November 28, 2022
Seller of Joint Health, Collagen Protein and More Warned for Drug Claims
On November 14, 2022, the FDA issued a warning letter to The Truth Company, LLC (parent company of Kinobody, LLC and UMZU, LLC) following inspection of the company’s websites which found statements about its Betaine, Immune, Redwood, Sensolin, Thyrite, zuRelief, Kino Aminos, Kino Collagen ...
Recalls & Warnings
August 11, 2022
Problems With Supplements on Amazon
More than 50% of 30 top-listed immune support supplements purchased on Amazon.com in May of last year were found to list ingredients that could not be found in them with testing, according to a recent report (Crawford, JAMA Network Open, 2022).
Recalls & Warnings
May 29, 2018
FDA Warns Seller of "Aromatase Inhibitor" Supplement
On May 18, 2018, the FDA issued a warning letter to Performance Nutrition Formulators LLC d.b.a. VMI Sports because its product Arimistane contains Androsta-3,5-Diene-7,17-Dione, an ingredient that the FDA considers to be a new drug and not a dietary supplement ingredient.
Recalls & Warnings
November 12, 2019
Libido Supplement for Men and Women Recalled
Update: (2/25/20) This recall has been expanded to include lots of an additional Med Man product, Bow and Arrow libido enhancer for men, highlighted below.
Recalls & Warnings
December 21, 2019
Men's Enhancement Supplement Recalled
On December 17, 2019, Motto International Corp all lots of Bull Platinum 30000, Stallion Platinum 30000, Rhino 7 Platinum 30000, and Panther Platinum 30000, to the consumer leve because FDA analysis has found they contain tadalafil.
Recalls & Warnings
June 22, 2021
Living Free Vitamins, Joint, Nerve and Other Supplements Recalled
On June 21, 2021, Bea Lydecker's Naturals, Inc. issued a recall of six Living Free brand supplements because the labels do not declare soy lecithin.
Recalls & Warnings
June 02, 2022
Walmart Inc. Recalls Joint Supplements With Omega-3, Glucosamine & Curcumin
On May 28, 2022, Walmart Inc. issued a voluntary recall of all lots of Artri Ajo King Joint Supplements after FDA laboratory analysis found the presence of undeclared diclofenac.
Recalls & Warnings
December 15, 2022
Beware of Supplements, Homeopathic Products Promoted to Treat Flu, Says FDA
On December 13, 2022, the FDA warned consumers to beware of supplements and homeopathic products, as well as nasal sprays, sanitizers and other products that promise to prevent or treat the flu.
Recalls & Warnings
March 06, 2019
FDA Warns Consumers Not to Use Certain Sexual Enhancement Supplements
On January 28, 2019, the FDA warned consumers not to buy or use the following sexual enhancement supplements were found to contain undeclared sildenafil and/or tadalafil.
Recalls & Warnings
January 22, 2019
FDA Warns Consumers Not to Use "The Silver Bullet"
On January 10, 2019, the FDA warned consumers not to buy or use the sexual enhancement supplement The Silver Bullet because it contains undeclared sildenafil and tadalafil.
Recalls & Warnings
January 13, 2019
Sexual Enhancement Supplement Recalled
On January 8, 2019, Happy Together, Inc. issued a recall of the sexual enhancement supplement Rhino 5k because FDA analysis found it to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
December 11, 2018
Sexual Enhancement Supplement Found to Contain Prescription Drug
On December 10, 2018, the FDA warned consumers that the sexual enhancement supplement On Demand contains undeclared sildenafil.
Recalls & Warnings
December 01, 2018
Sexual Enhancement Supplements Contain Prescription Drugs
The FDA recently warned consumers that the following sexual enhancement supplements were found to contain undeclared sildenafil and/or tadalafil. Use the links below to read the complete warning letter for each:
- Willy Go Wild
Recalls & Warnings
April 12, 2019
Sexual Enhancement Supplement for Men Recalled
On April 9, 2019, SD Import, LLC issued a recall of Aphrodisiac, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
April 08, 2019
Herbal "Coffee" for Sexual Enhancement Recalled
On March 22, 2019, Brian Richardson DBA "In Tha Pink" issued a recall of ground Kopi Jantan Tradisional [sic] Natural Herbs Coffee, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil and tadalafil.
Recalls & Warnings
May 16, 2020
CBD Oil Recalled, Risk of High Lead Exposure
On May 15, 2020, Summitt Labs issued a recall of one batch of KORE ORGANIC Watermelon CBD Oil Tincture due to contamination with lead. A random sample of this product tested by the Florida Department of Agriculture and Consumer Services found it to contain lead levels at 4.7 ppm.
Recalls & Warnings
October 22, 2014
Supplements Recalled Years Ago Remain on the Market, Still Contain Hidden Drugs
On October 21, 2014, a report in the Journal of the American Medical Association (JAMA) revealed that almost 10% of the supplements that have been recalled over the past few years are still on the market - and many still contain the hidden drugs which prompted their initial recall.
Recalls & Warnings
March 23, 2019
BLUEFUSION Capsules for Sexual Enhancement Recalled
On March 21, 2019, Ata Int. Inc. issued a recall of its BLUEFUSION capsules, which are promoted for sexual enhancement, because FDA analysis found them to contain sildenafil, tadalafil, desmethyl carbodenafil, dithiodesmethyl carbodenafil, scutellarin and daidzein.
Recalls & Warnings
May 07, 2019
"The Beast" Supplement for Men Recalled
On May 7, 2019, STIFF BOY LLC issued a recall of The Beast, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
September 04, 2018
Federal Court Shuts Down Maker of Sexual Enhancement Products
On August 30, 2018, the FDA announced that U.S.
Recalls & Warnings
May 15, 2019
Titanium 4000 Capsules for Men Recalled
On May 7, 2019, D.B.P. Distribution issued a recall of Titanium 4000, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil and tadalafil.
Recalls & Warnings
March 26, 2019
LEOPARD Miracle Honey Recalled
On March 22, 2019, USA LESS issued a recall of all lots of LEOPARD Miracle Honey, a supplement promoted for male sexual enhancement, because FDA analysis found it to contain sildenafil.
Recalls & Warnings
March 17, 2020
"Active Male" Supplement Recalled
On March 16, 2020, Natural Remedy Store recalled all lots of Active Male, 500mg, to the consumer level because FDA analysis has found the product contains tadalafil.
Recalls & Warnings
May 19, 2020
FTC Sends Refund Checks to Consumers of Unproven Weight Loss and Sexual Enhancement Supplements
On May 19, 2020, the FTC announced it is mailing 143,636 checks totaling more than $8,500,000 to consumers who purchased deceptively marketed supplements.
Recalls & Warnings
November 26, 2019
Cognitive Enhancement Supplements Contain Unapproved Drug
Several supplements sold in the U.S. and promoted for cognitive enhancement contain significant amounts of the drug piracetam -- a drug that is not approved for use in the U.S. -- according to a study published this week in JAMA Internal Medicine.
Recalls & Warnings
June 05, 2020
Seller of CBD, Sleep Aids, and Cold & Flu Products Warned for Manufacturing Violations
On April 28, 2020, the FDA issued a warning letter to The Dragontree Apothecary LLC, which found the company's Sleep Support, Anxiety Relief, Cold & Flu Relief, and Inflammation Relief to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
March 05, 2020
Protein Powder Recalled Due to Undeclared Milk
On March 3, 2020, New Capstone, Inc. issued a recall of ReStructure Vanilla Protein Powder because it may contain undeclared milk.
Recalls & Warnings
January 25, 2021
Clover Leaf Sardines Recalled Due to Risk of Botulism
On January 22, 2021, Clover Leaf Seafoods Corp.
Recalls & Warnings
May 29, 2021
FDA, FTC Warns Five Sellers of "Infertility" Supplements
The FDA and FTC (Federal Trade Commission) sent warning letters to the following five companies in May for illegally selling dietary supplements promoted with claims to treat infertility and other reproductive health issues:
Recalls & Warnings
November 20, 2020
FTC Files Complaint Against Two Supplement Companies for Deceptive Marketing
On November 20, 2020, the FTC approved a Part 3 administrative complaint against Health Research Laboratories, LLC, its owner Kramer Duhon, and Whole Body Supplements, LLC for making unverified claims that their products can prevent or treat diseases.
Recalls & Warnings
February 17, 2015
Seller of Muscle Supplements Containing Synthetic Steroids Warned
On February 9, 2015, the FDA issued a warning letter to A2Z Industries, LLC, following a facility inspection which found the company's muscle enhancing supplements, including HaloV and EPI2A3A to be labeled as containing synthetic steroids.
Recalls & Warnings
April 17, 2018
Sexual Enhancement Supplement Recalled
On April 16, 2018, Epic Products, LLC, recalled its sexual enhancement supplement Euphoric because it was found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
April 14, 2018
Men's Sexual Enhancement Supplement Recalled
On April 12, 2018, AMA Wholesale Inc. recalled its sexual enhancement supplement Rhino 69 Extreme 50000 because it was found to contain undeclared tadalafil.
Recalls & Warnings
June 25, 2020
"Anti-Viral" Supplement Recalled for Unsupported Coronavirus Claims
On June 23, 2020, Golden Nutrition Inc. issued a recall of four lots of Anti-Viral Immune Enhancement Capsules because the label makes unsupported health claims to "help fight corona virus and influenza.
Recalls & Warnings
September 08, 2020
Holistic Healthy Pet Warned for Drug Claims
On August 25, 2020, the FDA issued a warning letter to Savvy Holistic Health dba Holistic Healthy Pet following a review of the company's website, which found statements made about some of the company's products, including Doctor's Best for Your Pets, Savvy Holistic Health Pet Essences, ...
Recalls & Warnings
September 23, 2017
Enhancement Supplement "Vegetable Vigra" Recalled
On September 20, 2017, Gadget Island, Inc. Dba Gear Isle recalled VEGETABLE VIGRA [sic] after FDA testing found undeclared presence of sildenafil.
Recalls & Warnings
September 21, 2017
Sexual Enhancement Supplements Containing Prescription Drugs Recalled
On September 20, 2017, Gadget Island, Inc. Dba Gear Isle recalled the following supplements because they were found to contain undeclared sildenafil and tadalafil:
- Rhino 7 Platinum 5000 capsules, Lot# R7-D5K1011H
Recalls & Warnings
August 03, 2017
AMPT "Sexual Enhancement" Coffee Recalled
On August 1, 2017, AMPT Life, LLC, recalled all lots of AMPT Coffee after FDA testing found sildenafil and tadalafil.
Recalls & Warnings
December 19, 2017
Blue Pearl All Natural Enhancement Supplement Recalled
On December 13, 2017, Marmex Corp. recalled all lots of its sexual enhancement supplements Blue Pearl All Natural Male Enhancement Supplement, 500mg because they were found to contain undeclared sildenafil.
Recalls & Warnings
August 01, 2017
"Man of Steel" Sexual Enhancement Supplements Recalled
On July 27, 2017, Man of Steel recalled its Man of Steel 1 and Man of Steel 2 sexual enhancement supplements because they were found to contain undeclared sildenafil.
Recalls & Warnings
December 01, 2017
Nutra Labs Recalls Sexual Enhancement Supplements Containing Undeclared Drugs
On November 30, 2017, Nutra Labs Inc. recalled certain lots of its sexual enhancement supplements Bull 1800 mg Capsules and Chao Jimengnan 150 mg Tablets because they were found to contain undeclared sildenafil.
Recalls & Warnings
May 19, 2018
Sexual Enhancement Supplements Containing Undeclared Drugs Recalled
On May 17, 2018, Shoreside Enterprises, Inc., recalled certain lots of its sexual enhancement supplements 7K and Poseidon 4500 (Extreme 1000 mg) because they were found to contain undeclared sildenafil and/or tadalafil.
Recalls & Warnings
June 20, 2019
Weight Loss, Muscle & Energy Supplements Linked to Adverse Events in Children and Young Adults
Consumption of dietary supplements sold for weight loss, muscle building, and energy are associated with an increased risk for severe medical events in children and young adults compared to the consumption of vitamins, according to a recent study published in the Journal of the Adolescent ...
Recalls & Warnings
April 17, 2020
Seller of Vitamin D, MSM, Selenium & More Warned for Drug Claims
On March 31, 2020, the FDA issued a warning letter to KetoKerri LLC following a review of the company's website, which found statements made about some of the company's products, including Dr.
Recalls & Warnings
June 16, 2020
FDA Warns Four Companies for Unsafe "Homeopathic" Injectables
On June 16, 2020, the FDA issued warning letters to four manufacturers of unapproved injectable drugs labeled as homeopathic.
Recalls & Warnings
June 02, 2020
FTC Sends Refund Checks for "ReJuvination" Supplement
On June 1, 2020, the FTC announced it is mailing 1,310 checks totaling nearly $149,000 to consumers who purchased ReJuvination, a product deceptively marketed as a cure-all for various age-related conditions, including cell damage, heart attack damage, brain damage, and deafness.
Recalls & Warnings
May 30, 2020
FTC Sends Refund Checks for TrueAloe and AloeCran
On May 26, 2020, the FTC announced it is mailing 22,581 checks totaling more than $470,000 to consumers who purchased deceptively marketed supplements, TrueAloe and AloeCran.
Recalls & Warnings
June 23, 2020
FTC Sends Refund Checks to Consumers of Deceptive "Free Trial" Scheme
On June 22, 2020, the FTC announced it is mailing 187,425 checks totaling more than $8.
Recalls & Warnings
November 14, 2012
Maker of Vitamin C, Aloe and Sexual Enhancement Supplements Warned for Drug Claims and Misbranding
On October 22, 2012, the FDA issued a warning letter to Health Breakthroughs International, LLC after a review of the company's product labels and website found the dietary supplements Amazing C, MPS-Gold 100 and Power Herbal Formula to be promoted as drugs.
Recalls & Warnings
October 14, 2015
No Vinpocetine in Some Vinpocetine Supplements; Picamilon Labels Not Accurate
A recently published study of 23 supplements listing vinpocetine as an ingredient found six did not contain any vinpocetine. Among those that did contain vinpocetine, most did not list amounts on the label, but amounts found in suggested daily servings varied by almost 100-fold, from 0.
Recalls & Warnings
April 12, 2013
FDA Warns Consumers About The Dangers Of DMAA
On April 11, 2013, the FDA warned consumers about the dangers of dietary supplements containing the ingredient 1,3-dimethylamylamine (DMAA).
Recalls & Warnings
April 09, 2018
Muscle and Recovery Supplements Recalled Due to Undeclared Milk
On April 6, 2018, AdvoCare International issued a recall of two lots of Muscle Strength and four lots of Nighttime Recovery dietary supplements because they may contain undeclared milk.
Recalls & Warnings
June 23, 2020
BioPure Healing Products Warned for Manufacturing Violations
On June 17, 2020, the FDA issued a warning letter to BHP Holdings Inc.
Recalls & Warnings
January 24, 2015
FDA Warns Consumers Not To Use Sexual Enhancement Supplement
On January 21, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Happy Passengers, because it was found to contain undeclared sildenafil. Sildenafil, the active ingredient in Viagra, which is prescribed for erectile dysfunction.
Recalls & Warnings
December 26, 2014
Three Sexual Enhancement Supplements Found to Contain Hidden Drugs
On December 23, 2014, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain an undeclared drug.
Recalls & Warnings
February 19, 2019
Alaskan Smoked Silver Salmon in Jars and Cans Recalled Due to Risk of Botulism
On February 15, 2019, Smoked Alaska Seafoods, Inc. recalled all jars and cans of Smoked Silver Salmon because it has the potential to be contaminated with Clostridium botulinum, a bacterium which can cause life-threatening illness or death.
Recalls & Warnings
September 25, 2018
Seller of B Vitamins, Multis, Glucosamine & More Warned for Manufacturing Violations
On August 31, 2018, the FDA issued a warning letter to Independent Nutrition Inc., following a facility inspection which the company's products, including B-50 Complete, Multi-Vitamin & Mineral Complex (a.k.a.
Recalls & Warnings
September 25, 2018
FDA Warns Seller of Digestive Enzyme Supplements
On March 6, 2018, the FDA issued a warning letter to Uckele Health & Nutrition, Inc.
Recalls & Warnings
January 04, 2015
Sexual Enhancement Supplement Recalled
On December 30, 2014, Vivo Brand Management (Canada) issued a recall of one lot of the sexual enhancement supplement Forta for Men, because it was found to contain undeclared homosildenafil.
Recalls & Warnings
August 20, 2014
Sexual Enhancement Supplement Contains Hidden Drug
On August 11, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement Arize because it was found to contain sulfoaildenafil. Sulfoaildenafil is structurally similar to sildenafil, the active ingredient in Viagra, which is prescribed for erectile dysfunction.
Recalls & Warnings
August 16, 2014
FDA Warns Consumers Not To Use Herbal Sexual Enhancement Supplement
On August 11, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement Herbal Vigor Quick Fix because it was found to contain tadalafil. Tadalafil is the active ingredient in the prescription drug Cialis, which is prescribed for erectile dysfunction.
Recalls & Warnings
July 23, 2014
Sexual Enhancement Supplement Found to Contain Hidden Drug
On July 22, 2014, the FDA warned consumers not to buy or use sexual enhancement supplement O.M.G. because it was found to contain sildenafil.
Recalls & Warnings
June 18, 2014
Two Sexual Enhancement Supplements Found to Contain Drug
On June 17, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplements listed below because they were found to contain sildenafil.
Recalls & Warnings
June 06, 2014
Sexual Enhancement Supplement Found to Contain Drug
On June 5, 2014, the FDA warned consumers not to buy or use sexual enhancement supplement Zhen Gong Fu because it was found to contain sildenafil.
Recalls & Warnings
May 29, 2014
Recall of Sexual Enhancement Supplements Expanded
On May 28, 2014, Eugene Oregon, Inc. expanded its previous recall of certain lots of sexual enhancement supplements African Black Ant, Black Ant, and Mojo Risen. All lots of these supplements are now recalled.
Recalls & Warnings
May 16, 2014
Sexual Enhancement Supplement Contains Hidden Drug
On May 16, 2014, the FDA warned consumers not to buy or use sexual enhancement supplement MV5 Days because it was found to contain sildenafil.
Recalls & Warnings
May 07, 2014
Three Sexual Enhancement Supplements Recalled
On May 5, 2014 , Eugene Oregon, Inc. issued a precautionary, voluntary recall of sexual enhancement supplements African Black Ant, Black Ant and Mojo Risen.
Recalls & Warnings
April 18, 2014
Sexual Enhancement Supplement Contains Undeclared Drug
On April 16, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement S.W.A.G because it was found to contain sildenafil.
Recalls & Warnings
March 31, 2014
Seven Sexual Enhancement Supplements Recalled Due to Undeclared Drugs
On March 27, 2014, Nova Products, Inc. issued a voluntary recall of sexual enhancement supplements African Black Ant, Black Ant, XZen Gold, ZXen Platinum, XZen 1200, XZone Gold and XZone 1200.
Recalls & Warnings
March 07, 2014
Sexual Enhancement Supplement Found to Contain Undeclared Drug
On March 7, 2014, the FDA warned consumers not to buy or use the sexual enhancement supplement Weekend Warrior because it was found to contain undeclared thiosildenafil.
Recalls & Warnings
January 14, 2014
Seven Sexual Enhancement Supplements Containing Undeclared Drugs Recalled
On January 9, 2014, Midwest Wholesale issued a voluntary recall of seven sexual enhancement supplements: Boost Ultra, XZone Gold, Sexy Monkey, Triple MiracleZen Platinum, Magic for Men, "New" Extenze, and New XZen Platinum.
Recalls & Warnings
December 20, 2013
Two Sexual Enhancement Supplements Found to Contain Undeclared Drugs
On December 19, 2013, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain undeclared sildenafil or drugs chemically similar to sildenafil:
Recalls & Warnings
July 22, 2013
Sexual Enhancement Supplements Containing Undeclared Drugs Recalled
On July 18, 2013, Volcano Company issued a voluntary recall all lots of Volcano Male Enhancement Liquid and Volcano Male Enhancement Capsules because they were found to contain undeclared desmethyl carbodenafil, dimethylsildenafil, and dapoxetine.
Recalls & Warnings
March 06, 2015
Nine Male Enhancement Supplements Found to Contain Drugs
On March 2 and March 3, 2015, the FDA warned consumers not to buy or use the following nine male sexual enhancement supplements because they were found to contain an undeclared sildenafil or vardenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
March 02, 2015
Four More Sexual Enhancement Supplements Found To Contain Hidden Drug
On March 2, 2015, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain an undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
March 02, 2015
Hidden Drug Found in Six Sexual Enhancement Supplements
On February 27 and February 28, 2015, the FDA warned consumers not to buy or use the following six sexual enhancement supplements because they were found to contain an undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
September 03, 2013
Sexual Enhancement Supplements Containing Undeclared Drug Recalled
On August 27, 2013, Hardmenstore.com issued a voluntary recall of sexual enhancement supplements 72HP, Evil Root and Pro Power Max because they were found to contain undeclared sildenafil.
Recalls & Warnings
November 08, 2013
Sexual Enhancement Supplement Found To Contain Prescription Drugs
On November 8, 2013, the FDA warned consumers not to purchase or use sexual enhancement supplement Vitalikor Fast Acting because it was found to contain sildenafil and vardenafil.
Recalls & Warnings
October 01, 2013
Three Sexual Enhancement Supplements Containing Undeclared Drug Recalled
On September 24, 2013, Haute Health, LLC issued a voluntary recall of all lots of sexual enhancement supplements Virilis Pro, PHUK and Prolifta, because they were found to contain sildenafil.
Recalls & Warnings
September 27, 2013
Sexual Enhancement Supplement Containing Undeclared Drug Recalled
On September 24, 2013, the FDA warned consumers not to buy or use sexual enhancement supplement Wood-E because it was found to contain sildenafil. Sildenafil, the active ingredient in the prescription drug Viagra, is typically prescribed for erectile dysfunction.
Recalls & Warnings
November 20, 2013
Another Sexual Enhancement Supplement Recalled Due To Undeclared Drugs
On November 18, 2013, Fossil Fuel Products, LLC issued a voluntary recall of sexual enhancement supplement RezzRX because it was found to contain the undeclared drugs hydroxylthiohomosildenafil and aminotadalafil.
Recalls & Warnings
November 20, 2013
Two Sexual Enhancement Supplements Found To Contain Undeclared Drugs
On November 19, 2013, Tendex issued a voluntary recall of one lot each of sexual enhancement supplements P-Boost and NatuRECT because they were found to contain tadalafil.
Recalls & Warnings
January 30, 2014
Five Sexual Enhancement Supplements Found to Contain Prescription Drugs
On January 28, 2014, the FDA warned consumers not to buy or use the five sexual enhancement supplements listed below because they were found to contain sildenafil and/or tadalafil.
Recalls & Warnings
June 04, 2014
Six Sexual Enhancement Supplements Found to Contain Hidden Drugs
On June 2, 2014, the FDA warned consumers not to buy or use the six sexual enhancement supplements listed below because they were found to contain undeclared sildenafil and tadalafil, or other drugs which are chemically similar.
Recalls & Warnings
October 30, 2013
Three Sexual Enhancement Supplements Found to Contain Prescription Drugs
On September 24, 2013, the FDA advised consumers not to purchase or use sexual enhancement supplements Xzen 1200, Xzen Gold and Xzen Xpress because they have been found to contain undeclared prescription drugs.
Recalls & Warnings
November 21, 2013
Sexual Enhancement Supplement Found To Contain Multiple Drugs
On November 21, 2013, the FDA warned consumers not to buy or use sexual enhancement supplement Alpha Male because it was found to contain multiple undeclared drugs, including aminotadalafil, sulfosildenafil, sulfoaildenafil, hydroxythiohomosildenafil, dimethylsildenafil, and sildenafil.
Recalls & Warnings
November 20, 2013
Three Sexual Enhancement Supplements Recalled
On November 16, 2013, Jobbers Wholesale issued a voluntary recall of sexual enhancement supplements Rhino 5 Plus, Maxtremezen, and Extenzone because they were found to contain the undeclared drugs desmethylcarbondenafil and dapoxetine.
Recalls & Warnings
August 23, 2013
Sexual Enhancement Supplement Containing Undeclared Drugs Recalled
On August 12, 2013, Jack Rabbit Inc. issued a voluntary recall of one lot of sexual enhancement supplement Jack Rabbit because it was found to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
July 15, 2013
Two Sexual Enhancement Supplements Recalled
On July 5, 2013, Hardmenstore.com issued a voluntary recall of 430 lots of sexual enhancement supplements Silver Sword and Clalis because they were found to contain undeclared sildenafil.
Recalls & Warnings
June 28, 2013
Three Sexual Enhancement Supplements Found Contain Undeclared Drugs
On June 27, 2013, the FDA warned consumers not to buy or use MVP Mega, Exten 1300 and MaxTreme Zen, dietary supplements for sexual enhancement which were found to contain undeclared tadalafil or a combination of tadalafil and sildenafil.
Recalls & Warnings
April 22, 2015
FDA Warns Consumers Not to Use Male Enhancement Supplement
On April 21, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Extreme Diamond 3000 because it was found to contain desmethyl carbodenafil and dapoxetine.
Recalls & Warnings
March 11, 2015
Three Male Enhancement Supplements Found to Contain Drug
On March 3, 2015, the FDA warned consumers not to buy or use the following three sexual enhancement supplements because they were found to contain an undeclared sildenafil (each was identified during an examination of international mail shipments):
Recalls & Warnings
October 24, 2015
Drug Found in Male Enhancement Supplement
On October 23, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement S.W.A.G.G.E.R Extreme because it was found to contain sildenafil.
Recalls & Warnings
August 15, 2015
FDA Warns Seller of Tainted Enhancement Supplements
On July 31, 2015, the FDA issued a warning letter to R Thomas Marketing following a review of the company's website, which found it sold the male enhancement supplements Black Ant, Herb Viagra, Real Skill and Stree Overlord . These supplements contain the undeclared sildenafil.
Recalls & Warnings
July 01, 2015
Undeclared Drugs Found In Male Enhancement Supplements
On June 10, 2015, the FDA issued a warning letter American Lifestyle, following tests which found the company's sexual enhancement supplements Vicerex and Sudibil-Xr to contain the undeclared drugs propoxyphenyl thioaildenafil and tadalafil.
Recalls & Warnings
May 02, 2015
Three Sexual Enhancement Supplements Contain Prescription Drug
On April 30, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplements listed below because they were found to contain sildenafil. Each was identified during an examination of international mail shipments:
Recalls & Warnings
November 28, 2015
FDA Warns Consumers Not to Buy or Use "Sex Drive Capsules"
On November 19, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Sex Drive Capsules because they were found to contain sildenafil.
Recalls & Warnings
April 19, 2017
Sexual Enhancement Supplements for Men and Women Recalled
On April 18, 2017, Organic Herbal Supply, Inc. issued a recall of all lots of the following sexual enhancement supplements for men because they were found to contain the undeclared drug tadalafil:
Recalls & Warnings
June 27, 2013
Another Sexual Enhancement Supplement Found Contain Undeclared Drug
On June 27, 2013, the FDA warned consumers not to buy or use Silver sword, a dietary supplement for sexual enhancement which was found to contain undeclared sildenafil.
Recalls & Warnings
May 07, 2013
FDA Warns Consumers Of Three More Sexual Enhancement Supplements Containing Undeclared Drugs
On May 7, 2013, the FDA issued a warning to consumers, urging them not to purchase or use three sexual enhancement products which were found to contain undeclared drugs.
Recalls & Warnings
December 01, 2015
Enhancement Supplements Found to Contain Prescription Drug
On November 19, 2015, the FDA warned consumers not to buy or use the following sexual enhancement supplements, which were identified during an examination of international mail shipments, because they were found to contain undeclared sildenafil (click on the name of each supplement to read the ...
Recalls & Warnings
January 22, 2016
Male Enhancement Gum and Pills Contain Hidden Drug
On January 21, 2016, the FDA warned consumers not to buy or use the following sexual enhancement supplements, because they were found to contain undeclared vardenafil (click on the name of each supplement to read the FDA's complete warning):
Recalls & Warnings
March 06, 2015
Four Male Enhancement Supplements Found to Contain Drug
On March 5, 2015, the FDA warned consumers not to buy or use the following four sexual enhancement supplements because they were found to contain an undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
June 19, 2013
Sexual Enhancement Supplement Found To Contain Undeclared Drug
On June 17, 2013, the FDA advised consumers not to purchase or use sexual enhancement supplement Royal Dragon Herbal Tonic Balls because it was found to contain undeclared vardenafil.
Recalls & Warnings
June 13, 2013
FDA Warns Consumers of Sexual Enhancement Supplements Containing Prescription Drugs
On June 10, 2013, the FDA warned consumers that a number of sexual enhancement supplements have been found to contain undeclared drugs such as sildenafil and tadalafil.
Recalls & Warnings
May 08, 2013
Three Sexual Enhancement Supplements Recalled for Undeclared Drug Risk
On May 7, 2013, BeaMonstar Products issued a voluntary recall of sexual enhancement supplements SexVoltz and Velextra because they were found to contain an undeclared drug.
Recalls & Warnings
April 25, 2013
FDA Warns Consumers of Sexual Enhancement Supplements Containing Drugs
On April 25, 2012, the FDA issued a warning to consumers, urging them not to purchase or use two sexual enhancement products which were found to contain undeclared drugs.
Recalls & Warnings
March 21, 2013
FDA Warns Consumers: Three Sexual Enhancement Supplements Found To Contain Drugs
On March 21, 2013, the FDA warned consumers not to purchase or use three sexual enhancement dietary supplements, Rock-It Man, Libido Sexual Enhancer, and Stiff Days, because they were found to contain sildenafil or sildenafil analogues.
Recalls & Warnings
January 12, 2016
Twenty-four Male Enhancement Supplements Recalled
On January 9, 2016, R Thomas Marketing LLC. in conjuction with Just Enhance LLC, issued a recall of the following sexual enhancement supplements because they were found to contain sildenafil:
Recalls & Warnings
December 19, 2015
"Tiger X" and "X Again Platinum" Contain Hidden Drugs
On December 17 and 18, 2015, the FDA warned consumers not to buy or use the following sexual enhancement supplements, which were found to contain undeclared drugs:
Recalls & Warnings
October 24, 2015
Eight Enhancement Supplements Found to Contain Undeclared Drugs
On October 23, 2015, the FDA warned consumers not to buy or use the following three sexual enhancement supplements because they were found to contain an undeclared sildenafil or similar drugs (some were identified during an examination of international mail shipments):
Recalls & Warnings
February 21, 2017
Sexual Enhancement Supplement Recalled
On February 16, 2017, Organic Herbal Supply, Inc. issued a recall of its sexual enhancement supplement XtraHRD Natural Male Enhancement because it was found to contain tadalafil.
Recalls & Warnings
May 17, 2016
Sexual Enhancement Supplement Contains Undeclared Drugs
On May 6, 2016, the FDA issued a warning letter to Economax, LLC because the company's sexual enhancement supplement, Super Power Khan was found to contain sildenafil and hydroxyhomosildenafil.
Recalls & Warnings
July 18, 2017
Hidden Drugs Found in Sexual Enhancement Products
On July 17, 2017, the FDA posted public notifications after finding multiple sexual enhancement products contained hidden drug ingredients.
Recalls & Warnings
May 26, 2017
Herbal "Sexual Enhancement Coffee" Recalled, One Death Reported
On May 25, 2017, Caverflo.com issued a recall of Caverflo Natural Herbal Coffee, an "herbal" instant coffee promoted for sexual enhancement, because it was found to contain the drugs sildenafil and tadalafil.
Recalls & Warnings
May 15, 2016
Sexual Enhancement Supplements Recalled
On May 10, 2016, SOS Telecom, Inc. issued a recall of the following sexual enhancement supplements, which were found to contain undeclared sildenafil:
Recalls & Warnings
December 23, 2016
Twelve Sexual Enhancement Supplements Found to Contain Prescription Drug
On December 22, 2016, the FDA warned consumers not to buy or use the following twelve sexual enhancement supplements because they were found to contain undeclared sildenafil. Each was identified during an examination of international mail shipments.
Recalls & Warnings
December 12, 2015
Sexual Enhancement Supplement Recalled
On December 11, 2015, Reesna Inc. issued a recall of its sexual enhancement supplements Fuel Up Plus and Fuel Up High Octane which were distributed in August 2015 and were found to contain undeclared hydroxythiohomosildenafil.
Recalls & Warnings
September 16, 2015
Weight And Enhancement Supplement Recall Expanded
On September 11, 2015, One Minute Miracle Inc. issued a recall of all lots the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
April 10, 2013
Recall: Sexual Enhancement Supplement Containing Undeclared Drug
On April 1, 2013, Consumer Concepts, Inc. issued a voluntary nationwide recall of ROCK-It MAN Male Enhancement Capsules, a dietary supplement promoted for sexual enhancement which was found to contain undeclared hydroxythiohomosildenafil, an analogue of the prescription drug sildenafil.
Recalls & Warnings
December 19, 2013
Seller of Sexual Enhancement Supplement and Tea Drink Warned for Drug Ingredients and Drug Claims
On December 5, 2013, the FDA issued a warning letter to Green Planet, Inc. following a facility inspection which found the company's sexual enhancement supplement Night Bullet to contain the drugs sulfohydroxyhomosildenafil, thioaildenafil and aminotadalafil.
Recalls & Warnings
February 13, 2016
FDA Warns Nine Sexual Enhancement Supplements Contain Undeclared Drugs
The FDA recently warned consumers not to buy or use the following sexual enhancement supplements, which are sold on various websites and in some retail stores, because they were found to contain undeclared drugs:
Recalls & Warnings
July 22, 2017
Sexual Enhancement Supplement Containing Prescription Drugs Recalled
On July 21, 2017, Ultra Shop Supplement recalled Super Panther 7K capsules after FDA testing found them to contain undeclared sildenafil and tadalafil.
Recalls & Warnings
July 18, 2017
Herbal "Sexual Enhancement Coffee" Recalled
On July 13, 2017, Bestherbs Coffee LLC recalled their product New Kopi Jantan Tradisional Natural Herbs Coffee after FDA testing found desmethyl cabodenafil.
Recalls & Warnings
March 04, 2016
FDA Warns Consumers Not To Use Sexual Enhancement Supplement
On March 3, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Sextra because it was found to contain sildenafil.
Recalls & Warnings
February 27, 2016
Enhancement Supplement Contains Undeclared Drug
The FDA recently warned consumers not to buy or use the sexual enhancement supplement Neophase Natural Sex Enhancer because it was found to contain hydroxyacetildenafil.
Recalls & Warnings
November 07, 2015
Power Khan Herbal Enhancement Supplement Found to Contain Drug
On November 5, 2015, the FDA warned consumers not to buy or use the sexual enhancement supplement Power Khan because it was found to contain sildenafil.
Recalls & Warnings
December 29, 2016
Sexual Enhancement Supplement Contains Prescription Drug
On December 22, 2016, the FDA warned consumers not to buy or use the sexual enhancement supplement Power Male Sexual Stimulant because it was found to contain sildenafil. This supplement was identified during an examination of international mail shipments.
Recalls & Warnings
November 11, 2016
Male Enhancement Supplement Contains Hidden Drug
On November 9, 2016, the FDA warned consumers not to buy or use the sexual enhancement supplement Ready Man! because it was found to contain sildenafil and phenolphthalein.
Recalls & Warnings
September 26, 2015
Male Enhancement Supplement Containing Antidepressant Recalled
On September 25, 2015, TF Supplements issued a recall of two lots of the sexual enhancement supplement RHINO 7, which were found to contain the undeclared drugs desmethyl carbondenafil and dapoxetine.
Recalls & Warnings
October 14, 2015
Recall of Male Enhancement Supplement Expanded
On October 9, 2015, TF Supplements expanded its previous recall of two lots of RHINO 7 sexual enhancement supplements, which were found to contain the undeclared drugs desmethyl carbondenafil and dapoxetine, to include all lots.
Recalls & Warnings
April 17, 2015
Herbal Laxative and "Detox" Kit Containing High Levels of Lead and/or Arsenic Recalled in Canada
On April 15, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced that St. Francis Herb Farm Inc.
Recalls & Warnings
April 08, 2015
Does Your Weight Loss or Sports Supplement Contain Synthetic Amphetamine?
A study published on April 7, 2015, reported that among 21 weight loss, sports and cognitive enhancement supplements labeled as containing the plant extract Acacia rigidula, more than half were found to contain a synthetic, amphetamine-like compound called beta- methylphenylethylamine ...
Recalls & Warnings
October 09, 2014
New Synthetic Stimulant Found in Supplements
A synthetic stimulant never before studied in humans, 1,3-dimethylbutylamine (DMBA), was found in a dozen supplements sold by U.S. distributors in dosages from 13 to 20 mg per serving (Cohen, Drug Test Analys 2014).
Recalls & Warnings
May 03, 2013
Recall: Two Sexual Enhancement Supplements Containing Undeclared Drugs
On May 1, 2013, American Lifestyle issued a voluntary recall of all lots of sexual enhancement supplements Vicerex and Black Ant because they were found to contain undeclared prescription drugs.
Recalls & Warnings
January 02, 2016
Fourteen Enhancement Supplements Found to Contain Undeclared Drugs
On December 28, 2015, the FDA warned consumers not to buy or use the following fourteen sexual enhancement supplements, which are sold on various websites and in some retail stores, because they were found to contain undeclared drugs :
Recalls & Warnings
March 20, 2016
Salute Capsules Contain Hidden Drugs
On March 17, 2016, the FDA warned consumers not to buy or use the sexual enhancement supplement Salute Capsules because it was found to contain sildenafil, thiosildenafil, and sulfoaildenafil.
Recalls & Warnings
February 04, 2017
FDA Warns Four Sexual Enhancement Supplements Contain Prescription Drug
The FDA recently warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain undeclared sildenafil.
Recalls & Warnings
March 21, 2017
Twenty-one Sexual Enhancement Products Recalled
On March 7, 2017, A&H Focal Inc. issued a recall of the following sexual enhancement supplements because they were found to contain drugs such as sildenafil, tadalafil, vardenafil:
Recalls & Warnings
July 06, 2018
Prescript-Assist Probiotic Recall Expanded
On July 6, 2018, LL's Magnetic Clay, Inc., expanded its original recall of Prescript-Assist, a "broad spectrum probiotic and prebiotic" supplement, because it may contain undeclared almonds, crustaceans, dairy, casein, eggs, and peanuts.
Recalls & Warnings
June 29, 2018
Prescript-Assist Probiotic Recalled
On June 29, 2018, LL's Magnetic Clay, Inc., issued a recall of certain lots of Prescript-Assist, a "broad spectrum probiotic and prebiotic" supplement, because they may contain undeclared almonds, crustaceans, dairy, casein, eggs, and peanuts.
Recalls & Warnings
January 17, 2018
Joint and Pain Relief Product Contains Undeclared Drug
On January 17, 2018 Health Canada warned consumers that Linsen Double Caulis Plus, a supplement for joint pain, was found to contain the undeclared drug dexamethasone.
Recalls & Warnings
October 12, 2018
Organic Plant Protein Shake Recalled
On October 11, 2018, Forager Project issued a recall of certain Nuts and Vanilla — Organic Plant Protein Shakes because the products contain almond flour which is not declared on the label.
Recalls & Warnings
April 04, 2013
Three More Sexual Enhancement Supplements Found To Contain Undeclared Drugs
On April 3, 2013, the FDA warned consumers that the sexual enhancement supplements AFFIRM XL, Love Rider and Ninja Mojo were found to contain undeclared erectile dysfunction drugs. Consumers are urged to stop using these supplements immediately.
Recalls & Warnings
March 04, 2017
Sexual Enhancement Supplements Found to Contain Drugs
On March 3, 2017, the FDA warned consumers not to buy or use the following sexual enhancement supplements because they were found to contain undeclared drugs:
Recalls & Warnings
November 01, 2016
FDA Warns Consumers Not to Use Twelve Energy and Sexual Enhancement Supplements
The FDA recently warned consumers not to buy or use the following "energy" and sexual enhancement supplements, which are sold on various websites and in some retail stores, because they were found to contain undeclared drugs:
Recalls & Warnings
December 03, 2016
Male Enhancement Supplement Recalled
On November 29, 2016, MS Bionic, Inc. issued a recall of all lots of Megajex Natural Male Sex Enhancer capsules because they were found to contain tadalafil and sildenafil.
Recalls & Warnings
March 13, 2015
Three Weight Loss Supplements Found to Contain Drugs
The FDA recently warned consumers not to buy or use the following weight loss supplements because they were found to contain undeclared drugs. Each was identified during an examination of international mail shipments.
Recalls & Warnings
August 30, 2015
Weight and Enhancement Supplements Recalled
On August 27, 2015, One Minute Miracle Inc. issued a recall of one lot each the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
August 07, 2015
Male Enhancement and Weight Loss Supplement Containing Drugs Recalled
On August 6, 2015 Blue Square Market Inc. issued a recall of the following supplements which were found to contain undeclared drugs:
Recalls & Warnings
May 15, 2008
Feds Seek Millions from Seller of Enzyte Sexual Enhancement Supplement
According to an article in the Cincinnati Enquirer, on Wednesday, May 14, federal prosecutors told a U.S. District Court judge that the government could seek as much as $450 million in forfeitures from the sellers of Enzyte, a male sexual enhancement supplement.
Recalls & Warnings
April 03, 2012
"France T253" Enhancement Supplement Contains Hidden Drug
April 3, 2012: The U.S. Food and Drug Administration (FDA) is advising consumers not to purchase or use “France T253,” a product for sexual enhancement. This product is promoted and sold on various Web sites, such as ebay.com.
Recalls & Warnings
January 28, 2013
Recall: Male Sexual Enhancement Supplement Found to Contain Prescription Drug
On January 24, 2013, D&S Herbals, LLC, dba Freedom Trading, announced a voluntary recall of one lot of the male sexual enhancement dietary supplement Super Power because it was found to contain undeclared sildenafil.
Recalls & Warnings
October 17, 2015
"Herbal Viagra" Taken by Lamar Odom Was a Known Problem
It was reported this week that former NBA star, Lamar Odom, entered a coma after taking as many as 10 pills of the "herbal Viagra" formula Reload, along with cocaine.
Recalls & Warnings
June 22, 2017
FDA Finds Hidden Drug Ingredients in Sexual Enhancement Supplements
On June 22, 2017, the FDA warned consumers that the following sexual enhancement supplements were found to contain undeclared sildenafil, tadalafil and/or dapoxetine. Use the links below to read the complete warning letter for each:
- Triple Premium Zen Gold 1300 mg
Recalls & Warnings
February 03, 2016
Seller of Weight and Enhancement Supplements Warned For Drug Claims
On December 11, 2015, the FDA issued a warning letter to The One Minute Miracle, Inc., following a review of the company's website which found statements made about Miracle Diet 30 and Miracle Rock 48 to be drug claims.
Recalls & Warnings
November 06, 2009
FDA Warns that "Stiff Nights" Enhancement Supplement Contains Undeclared Drug
On November 5, 2009 the U.S. Food and Drug Administration (FDA) warned consumers that Stiff Nights, a product marketed as a dietary supplement for sexual enhancement, contains an ingredient that can dangerously lower blood pressure and is illegal.
Recalls & Warnings
December 14, 2010
FDA Warns Consumers to Avoid Man Up Now Capsules
On December 14, 2010, the U.S. FDA warned consumers not to use Man Up Now capsules, marketed as a dietary supplement for sexual enhancement, because they contain a variation of an active drug ingredient found in Viagra that can dangerously lower blood pressure.
Recalls & Warnings
March 13, 2013
Recall: Male Sexual Enhancement Supplement Containing Drugs
On March 12, 2013, Green Planet, Inc. issued a recall of one lot of the male sexual performance enhancer supplement Night Bullet because it was found to contain trace amounts of sulfohydroxyhomosildenafil and aminotadalafil, analogues of the prescription drug sildenafil.
Recalls & Warnings
May 10, 2013
Seller Of Sexual Enhancement and Hormonal Balance Supplements Warned For Manufacturing Violations
On April 26, 2013, the FDA issued a warning letter to Pristine Bay, L.L.C.
Recalls & Warnings
October 29, 2016
Steroid "Alternatives" Not Permitted In Dietary Supplements, FDA Warns
On September 27, 2016, the FDA issued a warning letter to Proprietary Wellness, L.L.C.
Recalls & Warnings
May 17, 2012
FDA Warns of Drug Ingredients in Three Sexual Enhancement Supplements
On May 16, 2012, the U.S. FDA advised consumers not to purchase or use the following three sexual enhancement products: Boost - Ultra Sexual Enhancement Formula (sold on www.boostultra.biz), Firminite (sold on www.firminite.com), and VMaxx Rx (sold on www.vmaxxrx.com).
Recalls & Warnings
February 16, 2012
Recall of Enhancement Supplement for Women -- Contains Drug
On Feb, 10, 2012, Regeneca, Inc. announced a voluntary nationwide recall of RegenArouse Natural Female Intimacy Enhancement. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a pink capsule sold individually in foil packets.
Recalls & Warnings
September 13, 2010
ExtenZe Enhancement Supplements Seized in Canada
On August 19, 2010, Health Canada (Canada's health ministry) seized the sexual enhancement supplements "Male Enhancement ExtenZe" and "Women ExtenZe" which were imported from the U.S. Although legal and widely sold in the U.S.
Recalls & Warnings
March 29, 2013
Prescription Drugs Found In Prostate and Sexual Enhancement Supplements
October 24, 2012 the FDA issued a warning letter to USA Far Ocean Group Inc./ Health & Beauty Group Inc. because a number of the company's dietary supplements were found to contain pharmaceutical drugs, or were promoted with statements that constitute drug claims.
Recalls & Warnings
June 23, 2017
FDA Warns Seller of Menopause, Sexual Enhancement, Prostate Supplements and More For Manufacturing Violations
On May 26, 2017 the FDA issued a warning letter to Star Health & Beauty LLC, following a facility inspection which found the company's products, including Nu Essentials Royal Jelly Capsules, NuMan Male Enhancement Capsules, Star's Male Potency Tonic, NuGen HP, She Max HP, and V Max ...
Recalls & Warnings
January 29, 2018
Seller of Male Enhancement, Prostate Supplements & More Warned for Drug Claims
On January 10, 2018, the FDA issues a warning letter to USA Labs AKA Power Source Distributors, Inc following a review of the company's website which found statements made about its products, including Maximum Male, Beta 300 (Beta Prosturol), Chromium Max 1000, DHEA, Healthy Cold-X and ...
Recalls & Warnings
June 18, 2016
Muscle Milk Protein Drinks Recalled
On June 17, 2016 HP Hood LLC issued a recall of the following Muscle Milk protein drinks because they have the potential for premature product spoilage:
Recalls & Warnings
March 14, 2014
Arthritis and Muscle Pain Supplement Recalled
On March 12, 2014, Pain Free By Nature issued a recall of Reumofan Plus tablets sold on the company's website, www.painfreebynature.com, because it was found to contain the active pharmaceutical ingredients methocarbamol and diclofenac.
Recalls & Warnings
August 28, 2013
Maker of Sexual Enhancement Supplement Warned for Manufacturing Violations
On July 11, 2013, the FDA issued a warning letter to Precise Nutrition International Inc.
Recalls & Warnings
May 17, 2013
Hoodia, Sexual Enhancement Supplements and More Found To Be Adulterated, Misbranded
On March 01, 2013, the FDA issued a warning letter to Desert Rose Manufacturing, Inc.
Recalls & Warnings
February 19, 2014
Seller of Sexual Enhancement Supplements Warned For Manufacturing Violations
On February 11, 2014, the FDA issued a warning letter to Maximus Niterider International Group, Inc.
Recalls & Warnings
December 17, 2010
FDA Steps Up Efforts to Battle Tainted Supplements
On Dec. 15, 2010, the Food and Drug Administration (FDA) took new steps aimed at keeping consumers safe from harmful products that are marketed as dietary supplements and that contain undeclared or deceptively labeled ingredients.
Recalls & Warnings
November 21, 2010
FDA Warns Consumers Not to Use Vigor-25
On November 19, 2010, the U.S.
Recalls & Warnings
September 18, 2012
Men’s Sexual Enhancement Supplement Containing Prescription Drug Recalled
On September 12, 2012, Body Basics Inc. issued a voluntary nationwide recall of ACTRA-Sx 500 Dietary Supplement Capsules after independent laboratory testing confirmed the supplement contains the prescription drug sildenafil citrate.
Recalls & Warnings
March 08, 2012
Rx Drugs Found in Weight Loss and Enhancement Supplements
On February 6, 2012, FDA issued a letter to Globe All Wellness, LLC warning that two of their products contain undeclared prescription drugs.
Recalls & Warnings
March 09, 2016
Four Sexual Enhancement Supplements Recalled
On March 7, 2016, Health Canada (the Canadian equivalent of the FDA) announced that Vivo Brand Management is recalling four sexual enhancement products sold in Canada which may contain the undeclared drug sildenafil. Some of these products also appear to be available for purchase in the U.S.
Recalls & Warnings
February 28, 2015
Drugs Found in Male Enhancement Supplements
On December 11, 2014, the FDA issued a warning letter to Biogenix USA, LLC, following a facility inspection which found the company's sexual enhancement products, HAM, CE6 and SARMZ to contain undeclared drugs, as well as drugs which were listed on the label but are not permitted ...
Recalls & Warnings
August 22, 2012
FDA Warns of Life-Threatening Side Effects and Withdrawal Symptoms from Two Arthritis, Muscle Pain Supplements
On August 21, 2012, the FDA issued a warning to consumers about the dangers of taking Reumofan Plus and Reumofan Plus Premium, two dietary supplements marketed for arthritis, osteoporosis and muscle pain.
Recalls & Warnings
April 14, 2015
Muscle Supplement Linked to Serious Liver Injury
On April 13, 2015, the FDA warned consumers not to use the muscle growth supplement Tri-Methyl Xtreme (distributed by Extreme Products Group) because it has been linked to serious liver injury.
Recalls & Warnings
April 06, 2018
Workout Supplements Recalled Due To Allergen Risk
On April 5, 2018, Independent Nutrition Inc, dba Back to Health of Eugene., issued a recall of certain lots of Ignite High Endurance Pre-Workout Supplements because they may contain undeclared milk.
Recalls & Warnings
February 27, 2018
Testosterone, Thyroid, Other Supplements Recalled Due to Undeclared Milk
On February 23, 2018, Progressive Laboratories, Inc., issued a recall of the following four supplements because they may contain undeclared milk:
Recalls & Warnings
November 01, 2017
FDA Warns Sellers of Bodybuilding Supplements Containing Steroid-Like Substances
On October 23, 2017, the FDA issued warning letters to three supplement distributors because several of their bodybuilding products contain selective androgen receptor modulators (SARMs).
Recalls & Warnings
July 27, 2012
Prescription and Street Drug Alternatives Found in Male Enhancement, Mood, and Sleep Supplements
On July 10, 2012, the FDA issued a warning letter to Evol Nutrition Associates, Inc. subsequent to a 2011 inspection which found a number of the company's products contained prescription and investigational drugs and were misbranded.
Recalls & Warnings
November 26, 2012
Manufacturer of Muscle Building Supplement Containing Steroid Pleads Guilty
On November 15, 2012, Myogenix Corp. pleaded guilty in federal court to knowingly manufacturing and distributing the dietary supplement Spawn, which contained the synthetic steroid Estradienedione/Estra/Tren. Estradienedione is a drug which is not approved by the FDA.
Recalls & Warnings
June 21, 2010
FDA Warns Parents to Be Careful Giving Vitamin D Drops to Infants
On June 15, 2010, the U.S. FDA (Food and Drug Administration) alerted parents and caregivers that some liquid Vitamin D supplement products are sold with droppers that could allow excessive dosing of Vitamin D to infants.
Recalls & Warnings
August 02, 2013
Vitamin B, Vitamin C and Multimineral Supplements Recalled Due To Anabolic Steroid Risk
On July 31, 2013, Purity First Health Products, Inc.
Recalls & Warnings
July 28, 2013
B Vitamin Supplement Found To Contain Anabolic Steroids
On July 26, 2013, the FDA warned consumers not to purchase or use Healthy Life Chemistry by Purity First B-50, a vitamin B dietary supplement which was found to contain anabolic steroids, methasterone and dimethazine.
Recalls & Warnings
February 13, 2014
Maker of Cholesterol and Workout Supplements Warned For Manufacturing Violations, Drug Claims and Unapproved Ingredient
On January 31, 2014, the FDA issued a warning letter to Exclusive Supplements Inc.
Recalls & Warnings
February 13, 2014
FDA Warns Consumers Not To Buy or Use Arthritis Supplement
On February 13, 2014, the FDA warned consumers not to buy or use arthritis supplement Arth-Q because it was found to contain ibuprofen.
Recalls & Warnings
January 16, 2014
Joint Health Supplement Contains Dangerous Drugs, FDA Warns
On January 15, 2014, the FDA warned consumers not to buy or use joint health supplement Pro ArthMax (by Human Science Foundation) because it was found to contain multiple drugs, including diclofenac, ibuprofen, naproxen, indomethacin, nefopam, and chlorzoxazone.
Recalls & Warnings
December 24, 2013
Muscle Growth Supplement Linked with Liver Failure
On December 23, 2013, the FDA advised consumers to immediately stop using muscle growth supplement Mass Destruction (by Blunt Force Nutrition) because it is labeled as containing at least one synthetic anabolic steroid and has been linked to at least one reported case of liver failure.
Recalls & Warnings
April 24, 2014
Arthritis Supplement Containing Multiple Drugs Recalled
On April 21, 2014, Nano Well-being Health Inc., issued a voluntary recall of two lots of arthritis supplement Super Arthgold, because it was found to contain the drugs chlorzoxazone, diclofenac and indomethacin.
Recalls & Warnings
July 09, 2009
Recall of Whey Protein Products Due to Possible Salmonella Contamination
On July 8, 2009, the U.S. FDA announced that Max Muscle Sports Nutrition issued a voluntary recall on July 3 of Max Muscle products containing whey protein concentrate due to potential Salmonella contamination.
Recalls & Warnings
February 13, 2013
FDA Warns Maker of Weight Loss and Whey Protein Supplements For Manufacturing Violations and Misbranding
January 25, 2013, the FDA issued a warning letter to dietary supplement manufacturer SciLabs Nutraceuticals, following a facility inspection which found the company's dietary supplements and supplement ingredients, including N-Large 2, Flo-Gard AB, Nitrogen Glutamine capsules, Nutritech nutritional ...
Recalls & Warnings
February 20, 2013
Recall: Arthritis and Muscle Pain Supplement Contains Prescription Drugs
On February 15, 2013, Reumofan Plus USA, LLC and Reumofan USA, LLC announced a recall of Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain, because it was found to contain the active pharmaceutical ingredients methocarbamol, dexamethasone, and diclofenac.
Recalls & Warnings
April 18, 2012
X-Rock Contains Undeclared Drug Ingredient
April 18, 2012: The Food and Drug Administration (FDA) is advising consumers not to purchase or use “X-Rock,” a product for sexual enhancement manufactured by CRM Laboratories and sold on various websites, including www.xrockme.com.
Recalls & Warnings
July 18, 2017
FDA Warns Seller of "Quick Slim with pure Hoodia Gardonii" and "Diabalance Herbal Blood Sugar Balance"
On July 11, 2017, the FDA issued a warning letter to Black Seed Herb, Inc.
Recalls & Warnings
April 09, 2003
Recall of Dangerous Sexual Enhancement Supplement Illegally Containing Viagra Ingredient
On April 4, 2003, the U.S. Food and Drug Administration's MedWatch program announced that Ultra Health Laboratories, Inc. and Bionate International, Inc. are warning consumers not to purchase or consume a product known as Vinarol tablets.
Recalls & Warnings
December 14, 2009
Nationwide Recall of Sexual Enhancement Supplements Containing Drug-like Compound
On December 14, 2009, the Federal Drug Administration (FDA) posted a notice on its website regarding the recall of many sexual enhancement supplements sold by Atlas Operations, Inc.
Recalls & Warnings
March 02, 2012
Recall of Enhancement Supplement for Men -- Contains Drug
On Feb, 24, 2012, Regeneca, Inc. announced a voluntary nationwide recall of all lots of RegenErect. The product was distributed throughout the U.S. and Puerto Rico to Internet consumers. The product is distributed as a blue capsule sold individually in foil packets with a UPC code of 816860010055.
Recalls & Warnings
December 19, 2012
Sexual Enhancement Supplements Recalled Due To Undeclared Drugs
On December 17, 2012, Performance Plus Marketing, Inc.
Recalls & Warnings
September 21, 2012
Sexual Enhancement Supplements For Men and Women Recalled Due to Prescription Drug Risk
On August 23, 2012, Evol Nutrition Associates Inc./Red Dawn issued a voluntary recall of Mojo Nights after FDA testing revealed the male sexual enhancement supplement contains undeclared prescription drugs tadalafil and sildenafil.
Recalls & Warnings
July 26, 2012
Sexual Enhancement Supplements Containing Prescription Drug Recalled
On July 20, 2012, CRM Laboratories issued a voluntary recall of all X-Rock 3 Day Pill For Men and Z-Rock All Natural Male Supplements after FDA testing found these products contain the drugs sildenafil and hydroxythiohomosildenafil.
Recalls & Warnings
November 21, 2013
Weight Loss Supplement Recalled
On November 14, 2013, Deseo Rebajar Inc. issued a recall of one lot of weight loss supplement Adipotrim XT because it was found to contain undeclared fluoxetine.
Recalls & Warnings
November 20, 2013
Weight Loss Supplement Found to Contain Drugs
On November 19, 2013, the FDA warned consumers not to buy or use weight loss supplement Slim Max because it was found to contain sibutramine and phenolphthalein.
Recalls & Warnings
November 12, 2013
Weight Loss Supplement Found To Contain Drugs
On November 8, 2013, Health Canada warned consumers of a number of weight loss products that have been found to contain sibutramine or phenolphthalein, including Paiyouji Natural Slimming Capsules, which may be available for purchase to U.S. consumers through online retailers.
Recalls & Warnings
November 07, 2013
FDA Warns Consumers of Weight Loss Supplement Containing Multiple Drugs
On November 7, 2013, the FDA warned consumers not to buy or use Jimpness Beauty Fat Loss Capsules because they were found to contain sibutramine, phenolphthalein, and sildenafil.
Recalls & Warnings
May 06, 2015
Joint Pain Supplements Found to Contain Diuretics, Antihistamines and Other Undeclared Drugs
On May 4, 2015, the FDA warned consumers not to buy or use the following supplements promoted for joint or back pain because they were found to contain undeclared drugs. Each was identified during an examination of international mail shipments.
Recalls & Warnings
May 23, 2013
Seller of Sexual Enhancement, Cholesterol, Resveratrol Supplements and More Warned For Drug Claims
On May 2, 2013, the FDA issued a warning letter to Alternative Health Supplements, following a review of the company's website, which found statements made about several dietary supplements, including Regenerect, Alligin, Astaxanthin Advantage, HDL Cholesterol Management, Resveratrol, Coral Calcium ...
Recalls & Warnings
June 04, 2012
FDA Warning on Supplement for Pain Relief
On June 1, 2012, the U.S. FDA warned consumers that Reumofan Plus, marketed as a “natural” dietary supplement for pain relief and other serious conditions, contains several active pharmaceutical ingredients not listed on the label that could be harmful.
Recalls & Warnings
August 09, 2007
FDA Warning on Red Yeast Rice Products
On August 9, 2007, the U.S. Food and Drug Administration (FDA) warned consumers not to buy or eat three red yeast rice products promoted and sold on Web sites. The products may contain an unauthorized drug that could be harmful to health.
Recalls & Warnings
December 02, 2014
Seller of Energy & Joint Supplements, Aloe, Silver and More Warned for Drug Claims
On November 24, 2014, the FDA issued a warning letter to Jansen Enterprises, LLC, dba HealthWorksUSA, following an inspection of the company's website which found statements made about Nutra Blast Natural Energy, Ionic Silver Water, Nutra Complete, and Nutra Gel to be drug claims.
Recalls & Warnings
November 21, 2014
Herbal Arthritis Supplement Found to Contain Undeclared Drugs
On November 20, 2014, the FDA warned consumers not to purchase or use the herbal dietary supplement Feng Shi Ling because it was found to contain the undeclared drugs diclofenac and indomethacin.
Recalls & Warnings
August 22, 2015
Synthetic Amphetamine, Less Protein and More Sugar Than Claimed Found in Workout Supplements
On August 7, 2015, the FDA issued a warning letter to New Dawn Nutrition, Inc.
Recalls & Warnings
June 06, 2015
Joint Supplement Contains Drugs, FDA Warns
On June 5, 2015 the FDA warned consumers not to buy or use joint pain supplement Pyrola Advanced Joint Formula because it was found to contain diclofenac and chlorpheniramine.
Recalls & Warnings
April 29, 2015
Teen Use of Weight Loss and Workout Supplements Labeled "For Adult Use Only" Concerning
Three new studies have raised concerns that teenagers are able to purchase "body-shaping" supplements, such as creatine, testosterone boosters, and "fat-burning" products from health food stores despite the fact that many of these supplements are labeled "for adult use only.
Recalls & Warnings
April 29, 2015
FDA Targets Weight Loss and Workout Supplements Listing Synthetic Stimulant DMBA
On April 24, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called 1,3-dimethylbutylamine (DMBA) on product labels.
Recalls & Warnings
November 29, 2016
Seller of Mineral, Joint Supplements and More Warned for Manufacturing Violations, Drug Claims
On July 1, 2016, the FDA issued a warning letter to BioGenyx-Basic Reset, following a facility inspection which found the company's products, including Ionyte, Aqualyte, Beta Factor, Bee Gold and Primo Java to be adulterated because they were prepared, packed, or held under ...
Recalls & Warnings
July 11, 2017
FDA Warns Dr. Carolyn Dean of Drug Claims Made for Magnesium, Calcium & Other Mineral Supplements
On June 27, 2017, the FDA issued a Warning Letter to Dr. Carolyn Dean of New Capstone, Inc.
Recalls & Warnings
May 06, 2017
Herbal Teas Recalled Due To Botulism Risk
On May 1, 2017, U.S. Deer Antler Ex. & Imp. of Los Angeles, California, issued a recall of various herbal teas because they have the potential to be contaminated with Clostridium botulinum. This bacterium can cause botulism, a potentially fatal form of food poisoning.
Recalls & Warnings
February 20, 2013
FDA Warns USPLabs For Adulteration and Drug Claims
On December 4, 2012 the FDA issued a warning letter to USPLabs, LLC following facility inspections which found the company's products, including dietary supplements Jacked3d, OxyElite Pro, Prime, and Super Cissus, to be adulterated because they were prepared, packed, or held under conditions that ...
Recalls & Warnings
February 07, 2013
Growth Factor Supplement For "Size, Strength and Stamina" Recalled Due To Potential Contamination
On January 2, 2013, EonNutra, LLC issued a voluntary recall of specific batches of Soto Supplements Growth Factor Complex 200 GFC 200 (2 fl oz.) liquid dietary supplement due to potential bacterial contamination.
Recalls & Warnings
January 23, 2013
USPLabs Settles Class Action Lawsuit Over Controversial DMAA Ingredient
On December 18, 2012, a settlement was approved in the class action lawsuit against USPLabs, which alleged the company's use of the ingredient DMAA (1,3-dimethylamine) in the supplements OxyELITEPRO and Jacked3d was not safe or legal.
Recalls & Warnings
April 16, 2012
Protein Supplement Maker Fails FDA Audit -- Many Products Affected
The FDA sent a Warning Letter (dated April 2, 2012) to Theta Brothers Sports Nutrition, Inc. regarding numerous violations of good manufacturing practices at its Lakewood, New Jersey facility.
Recalls & Warnings
October 27, 2013
Ingredient In Workout Supplements May Be Unsafe, FDA Warns
On October 11, 2013, the FDA directed USP Labs, LLC to cease distribution of workout supplement OxyElite Pro and and muscle-building supplement VERSA-1, which are labeled as containing the ingredient aegeline, or N-[2-hydroxy-2(4-methoxyphenyl) ethyl]-3-phenyl-2-propenamide.
Recalls & Warnings
September 15, 2013
Creatine Powder Containing DMAA Recalled
On September 12, 2013, Ge Pharma, LLC issued a recall of grape and fruit punch flavors of Creafuse Powder because they contain 1,3 dimethylamylamine (DMAA).
Recalls & Warnings
October 25, 2013
Supplement Company Warned For Numerous Manufacturing Violations
On August 2, 2013, the FDA issued a warning letter to DNE Nutraceuticals, Inc., following a facility inspection which found the company's products to be adulterated because they were packed, or held under conditions that violate Current Good Manufacturing Practices for dietary supplements.
Recalls & Warnings
December 19, 2013
Maker of Joint Health Supplements Warned for Manufacturing Violations
On December 4, 2013, the FDA issued a warning letter to PurQuality, LLC.
Recalls & Warnings
March 19, 2014
Maker of Herbal Capsules and Extracts Warned for Manufacturing Violations
On September 19, 2013, the FDA issued a warning letter to Herbalist and Alchemist, Inc.
Recalls & Warnings
April 03, 2014
Maker of Green Coffee Bean Extract and Weight Loss Supplement Warned for Manufacturing Violations
On March 13, 2014, the FDA issued a warning letter to Libi Labs, Inc.
Recalls & Warnings
April 21, 2012
FDA Finds Prescription Drug Analog in 3 Enhancement Supplements
On April 20, 2012, the U.S. FDA advised consumers not to purchase or use the following supplements from Enhance Nutraceutical: "Instant Hard Rod," "RigiRx Plus," and "Zen Maxx." FDA laboratory analysis confirmed that each contains aminotadalafil.
Recalls & Warnings
October 22, 2010
Enzyte "Male" Supplement Found to Affect Heart
The journal Archives of Internal Medicine (August 2010) includes a report of a small clinical trial of the dietary supplement Enzyte, which is marketed for "male enhancement."
Recalls & Warnings
October 08, 2010
"Red Flags" for Spotting Tainted Supplements
In recent years, the FDA has identified hundereds of products marketed as dietary supplements or conventional foods with hidden drugs and chemicals. In reaction to this, the FDA recently created a flyer to help retailers spot questionable products. ConsumerLab.
Recalls & Warnings
July 20, 2010
Recall of ED Supplement Containing Drug
On July 20, 2010, the U.S. FDA posted a news announcement from Good Health, Inc. regarding the recall of Vialipro, a sexual enhancement supplement sold nationally.
Recalls & Warnings
December 30, 2007
FDA Warns Consumers Not to Use Several "Shangai" Sexual Enhancement Supplements
On December 28, 2007, the U.S. Food and Drug Administration (FDA) advised consumers not to buy or use Super Shangai, Strong Testis, Shangai Ultra, Shangai Ultra X, Lady Shangai, and Shangai Regular, also marketed as Shangai Chaojimengnan, products.
Recalls & Warnings
July 23, 2009
Six Male Enhancement Supplements Found Adulterated
On July 15, 2009, the U.S. FDA announced that Obteron 1 Inc. dba Nature & Health Co. is recalling the following supplements: LibieXtreme, Y-4ever, Libimax X Liquid, Powermania Capsule, Powermania Liquid, and Herbal Disiac.
Recalls & Warnings
August 05, 2016
Seller of Fertilix Warned By FDA
On July 22, 2016, the FDA issued a warning letter to CellOxess LLC, following a review of the company's websites, social media pages and marketing literature which found statements made about Fertilix Preconceptual, Fertilix Low Dose and Fertilix Max to be drug claims.
Recalls & Warnings
July 23, 2016
Seller of GlucoCor Warned for Drug Claims
On June 30, 2016, the FDA issued a warning letter to MC-COR, LLC, following a review of the company's website and Facebook page which found statements made about its GlucoCor capsules to be drug claims.
Recalls & Warnings
October 25, 2016
Seller of Liquid Aloe & Mineral Supplement Warned for Manufacturing Violations
On August 10, 2016, the FDA issued a warning letter to Perfect Source Natural Products Inc.
Recalls & Warnings
March 08, 2016
Maker of Calcium and Vitamin C Supplements Warned for Manufacturing Violations
On September 17, 2015, the FDA issued a warning letter to Raphah, Inc.
Recalls & Warnings
December 05, 2015
FDA Warns Companies Selling Products Containing Picamilon
On November 30, 2015, the FDA issued warning letters to five supplement companies selling products containing picamilon, which is not a lawful dietary supplement ingredient in the U.S.
Recalls & Warnings
September 16, 2015
Herbal Extracts Recalled
On September 15, 2015, Iowa Select Herbs, LLC issued a recall of numerous herbal exacts following a permanent injunction which required the company to stop selling supplements.
Recalls & Warnings
April 03, 2015
Health Canada Suspends Sales of Male Fern Products
On April 2, 2015, Health Canada (the Canadian equivalent of the U.S. FDA) announced it has suspended the licenses of the natural health products containing the ingredient male fern (Dryopteris filix-max), due to concern over the safety of the ingredient when taken at higher doses.
Recalls & Warnings
September 23, 2014
Maker of Joint and Weight Products Warned for Hidden Drugs, Manufacturing Violations
On September 15, 2014, the FDA issued a warning letter to West Coast Laboratories, Inc., because the company's joint health supplements, Super ArthGold and Pro ArthMax, were found to contain hidden drugs.
Recalls & Warnings
April 26, 2015
FDA Identifies More Products Listing Synthetic Amphetamine
On April 22, 2015, the FDA issued warning letters to sellers of weight loss and workout supplements that list a synthetic, amphetamine-like compound called beta- methylphenylethylamine (BMPEA) on product labels.
Recalls & Warnings
October 01, 2016
Homeopathic Teething Tablets and Gels May Pose Danger, FDA Warns
On September 30, 2016, the FDA warned consumers that homeopathic teething tablets and gels may be dangerous for infants and children. The agency has received adverse events reports, including seizures, associated with the use of these products.
Recalls & Warnings
May 23, 2016
Amino Acid Supplements Recalled
On May 18, 2016, Ultimate Nutrition Inc. issued a recall of its Amino Gold amino acid tablets and capsules because they contain undeclared milk in the form of hydrolyzed whey protein.
Recalls & Warnings
March 05, 2014
Seller of Aloe and Vitamin C Supplements Warned for Manufacturing Violations
On December 12, 2013, the FDA issued a warning letter to Health Breakthroughs International, following a facility inspection which found the company's products, MPS Gold 100, MPS Gold 3X and Amazing C, to be adulterated because they were prepared, packed, or held under conditions that violate ...
Recalls & Warnings
August 23, 2013
FDA Warns Consumers of Supplement Containing Prescription Anti-Inflammatory Drug
On August 22, 2013, the FDA warned consumers not to use or purchase the dietary supplement Ortiga, because it contains the prescription drug ingredient, diclofenac.
Recalls & Warnings
March 01, 2013
Manufacturer of Joint, Weight Loss, Muscle Supplements and More Warned for Adulteration, Misbranding and Drug Claims
On February 4, 2013, the FDA issued a warning letter to DC Nutrition, Inc.
Recalls & Warnings
April 19, 2013
Recall: Protein Powder May Have Been Contaminated During Packaging
On March 14, 2013, F.H.G Corporation, dba Integrity Nutraceuticals, issued a voluntary recall of MusclePharm Cookies 'N" Cream Combat Powder and Banana Cream Combat Powder protein powders because they may be contaminated with extraneous materials due to problems on the packaging line.
Recalls & Warnings
April 11, 2008
Update: Hazardous Levels of Selenium Confirmed by FDA in Two Supplements
On April 11, 2008, the FDA notified healthcare professionals and patients that it has found hazardous levels of selenium in samples of certain flavors of the dietary supplement products "Total Body Formula" and "Total Body Mega Formula.
Recalls & Warnings
March 28, 2008
FDA Warns of Adverse Reactions with Two Liquid Supplements -- Selenium Toxicity Possible
The U.S. Food and Drug Administration is advising consumers not to purchase or consume Total Body Formula in the flavors of Tropical Orange and Peach Nectar, or Total Body Mega Formula in the Orange/Tangerine flavor.
Recalls & Warnings
January 16, 2010
Recall of Body Building Supplements Containing Steroids
On January 15, 2010, the Food and Drug Administration (FDA) posted a voluntary recall notice from MuscleMaster.com, Inc. regarding all lots and expiration dates of seventeen dietary supplements sold in 2009 from June 1 and Novemember 17.
Recalls & Warnings
July 29, 2009
FDA Warns Against Body Building Supplements with Steroid-like Compounds
On July 28, 2009, the U.S. FDA notified the public about new safety information concerning products marketed for body building and increasing muscle mass.
Recalls & Warnings
January 02, 2004
Canada Reiterates Warning on Products Containing Kava
On December 23, 2003, Health Canada reminded Canadian consumers of the serious risks associated with the use of products containing kava.
Recalls & Warnings
August 31, 2012
Energy, Fat Loss Supplement Recalled For Ephedrine Alkaloids
On August 28, 2012, dietary supplement re-sale distributor Brand New Energy (BNE) issued a recall of EphBurn 25 after FDA testing found the product to contain ephedrine alkaloids.
Recalls & Warnings
January 16, 2013
Nutrition Bars Recalled Due To Undeclared Allergen
On January 15, 2012, Belmont Confections Inc. issued a voluntary recall of Dymatize Nutrition Elite Gourmet Cookies & Cream bars and Dymatize Nutrition Elite Gourmet Fudge Brownie bars because they may contain undeclared peanuts.
Recalls & Warnings
December 21, 2012
Dangerous Supplement Being Sold Under New Name
On December 21, 2012, the FDA warned consumers that Reumofan Plus, a dietary supplement promoted for arthritis and muscle pain that has been linked to serious and sometimes fatal side effects, is now being sold under the name WOW.
Recalls & Warnings
June 01, 2012
Homeopathic Manufacturer Settles False Advertising Suit
June 1, 2012 — A federal court in California recently authorized the settlement of a class action lawsuit against Boiron Inc. and Boiron USA, Inc.





